(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on PSMA for diagnostics and treatment, and a presentation by Dr. Matthew Smith discussing the patients that should receive chemotherapy before PSMA radioligand therapy. Docetaxel, cabazitaxel, and 177Lu-PSMA-617 improve overall survival in metastatic castration-resistant prostate cancer (mCRPC), however, there is limited information about the comparative effectiveness of taxanes and PSMA radioligand therapy. Thus, the optimal sequencing of taxanes and PSMA radioligand therapy is undefined. Dr. Smith notes that studies from 20 years ago (TAX3271and SWOG 99162) suggest that docetaxel improves overall survival in mCRPC:
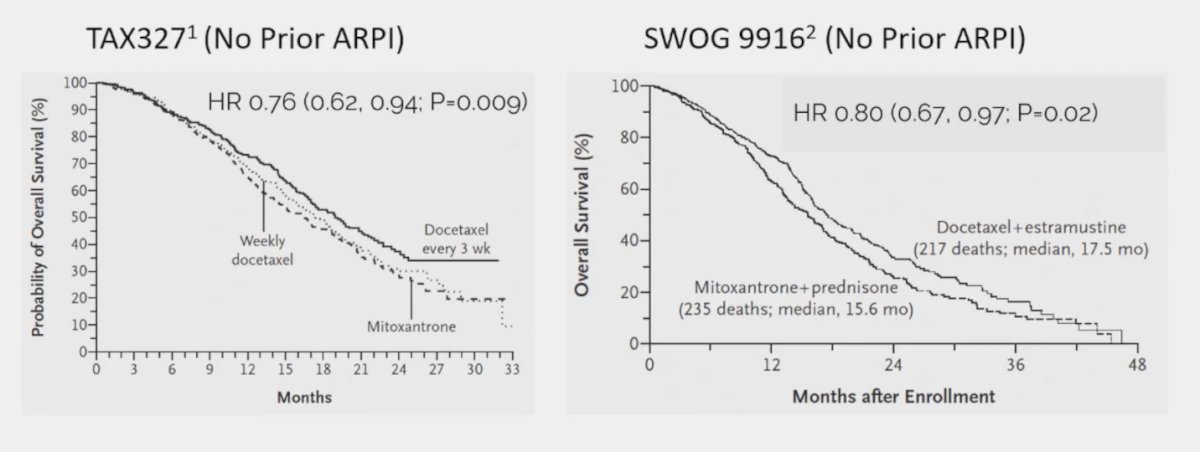
However, using a cross-trial comparison approach to assess the activity of docetaxel before and after an ARPI, Dr. Smith notes that docetaxel before ARPI (TAX327, SWOG 9916) shows a >= 50% decrease in PSA in 45-50% of patients and only 25% PSA reduction for docetaxel after enzalutamide in PRESIDE.3 Cabazitaxel also improves overall survival in mCRPC based on data from TROPIC4 (after docetaxel) and CARD5 (after ARPI and docetaxel) trials:
Third, 177Lu-PSMA-617 improves overall survival in mCRPC based on data from VISION6 (after ARPI and taxane chemotherapy), but with no survival benefit yet in PSMAfore (after ARPI, before taxane chemotherapy):
In an updated press release from Novartis on April 4, 2024, regarding PSMAfore, they note that “updated overall survival results from a pre-planned analysis at approximately 75% information fraction demonstrates an overall survival hazard ratio less than 1.0 in the intent-to-treat population, unadjusted for cross-over.” Dr. Smith notes that in a comparison of VISION and PSMAfore assessing 177Lu-PSMA-617 before and after taxane chemotherapy, the results for radiographic progression-free survival, objective response rate, and >= 50% PSA decrease are quite similar and that perhaps the major differences in overall survival are indeed secondary to the large crossover effect in PSMAfore:
In a comparison of PRESIDE and PSMAfore assessing docetaxel versus 177Lu-PSMA-617 in mCRPC after ARPI, there is a higher objective response rate, >=50% PSA decrease, and median radiographic progression-free survival in the PSMAfore cohort for 177Lu-PSMA-617:
Comparing CARD and VISION assessing cabazitaxel versus 177Lu-PSMA-617 after docetaxel and ARPI, we see very similar results for overall survival, radiographic progression-free survival, objective response rate, and >=50% PSA decrease: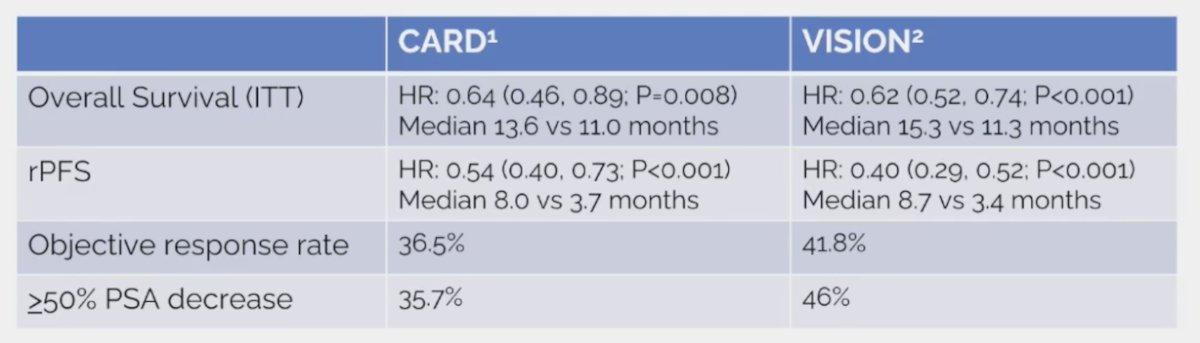
Looking at the VISION subgroup analysis for overall survival, it appears that patients with liver metastasis do quite poorly with 177Lu-PSMA-617 after docetaxel (HR 0.87, 95% CI 0.53 – 1.43), although Dr. Smith emphasized that these patients would likely do poorly with any therapy.
Dr. Smith states that the best comparison of chemotherapy and 177Lu-PSMA-617 comes from the TheraP trial.6 This was the first randomized study to evaluate 177Lu-PSMA-617 vs cabazitaxel for men with mCRPC after docetaxel. In this open-label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥ 20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥ 20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. The trial schema for TheraP is as follows: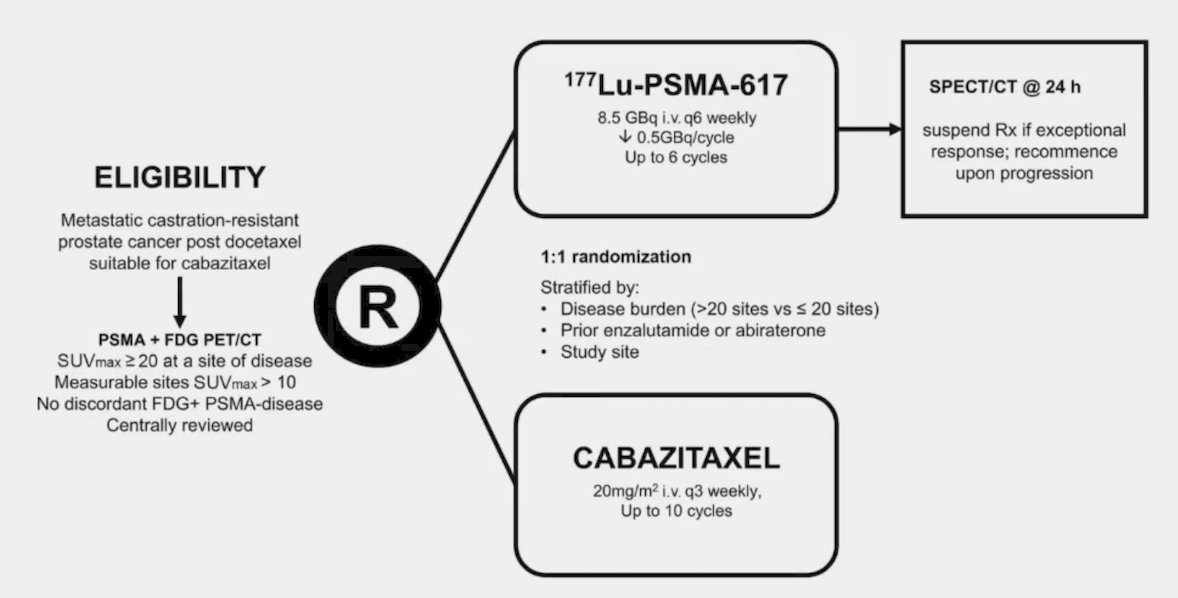
The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA-PFS and overall. After a median follow-up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86):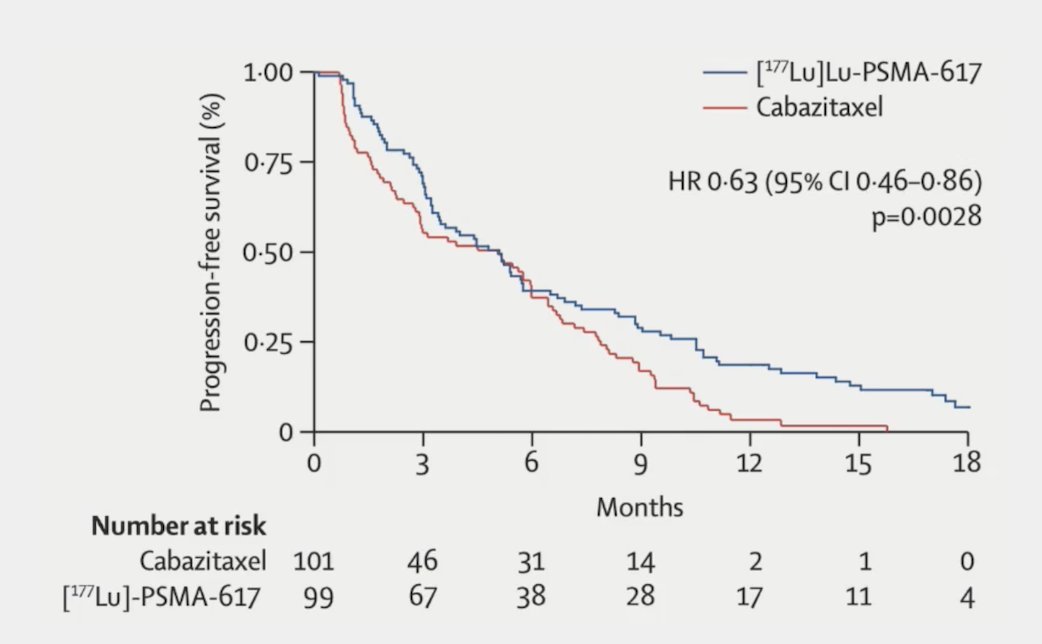
Additionally, 177Lu-PSMA-617 had a much higher PSA50 rate (66% vs 37%), particularly for those with SUVmean >= 10: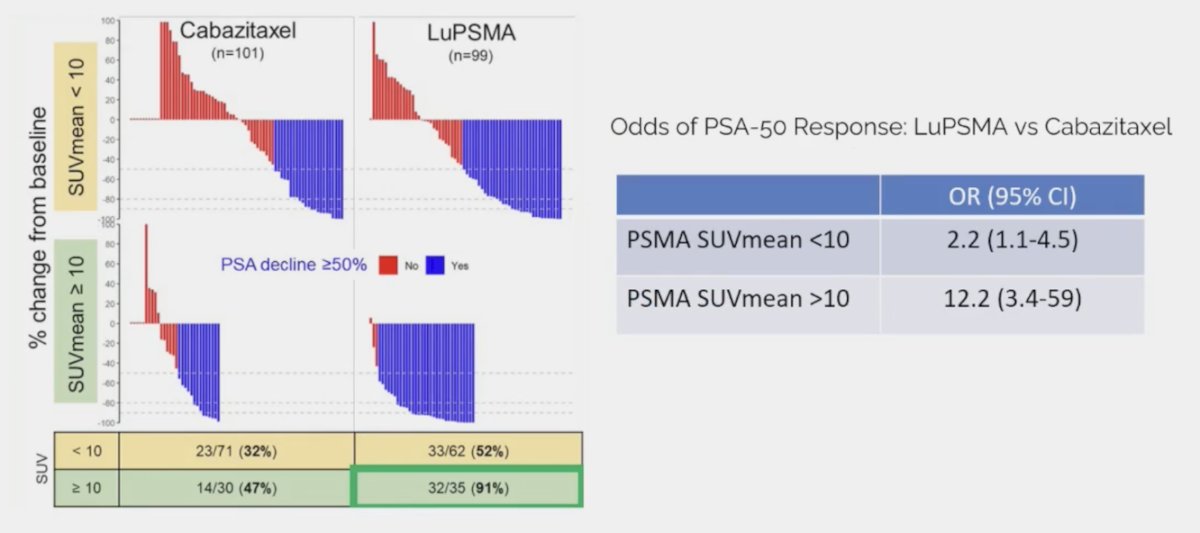
In work published in early 2024 in Lancet Oncology, Hofman and colleagues7 noted that after a median follow-up of 35.7 months (IQR 31.1 to 39.2), 77 (78%) participants had died in the 177Lu-PSMA-617 group and 70 (69%) participants had died in the cabazitaxel group. Overall survival was similar among those assigned to 177Lu-PSMA-617 versus those assigned to cabazitaxel (restricted mean survival time 19.1 months vs 19.6; difference -0.5 months; p = 0.77):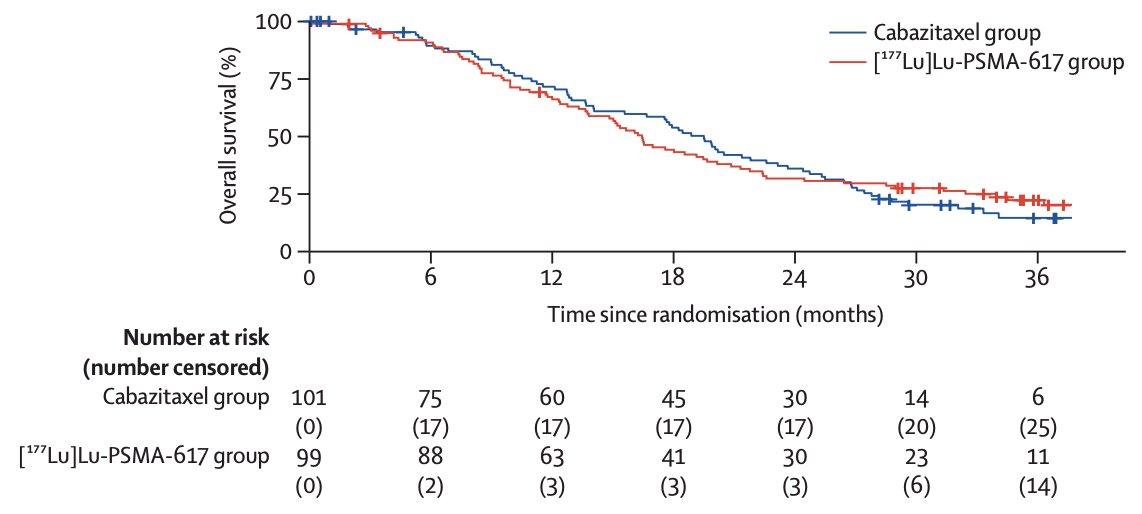
Moreover, there was no difference in overall survival when stratified by SUVmean +/- 10, in fact, cabazitaxel appears to do better in patients with SUVmean >= 10:
Several other key subgroup analyses were performed on this updated report of TheraP. First, patients with higher SUVmean (>= 10 vs < 10) had improved overall survival whether they were treated with cabazitaxel or 177Lu-PSMA-617: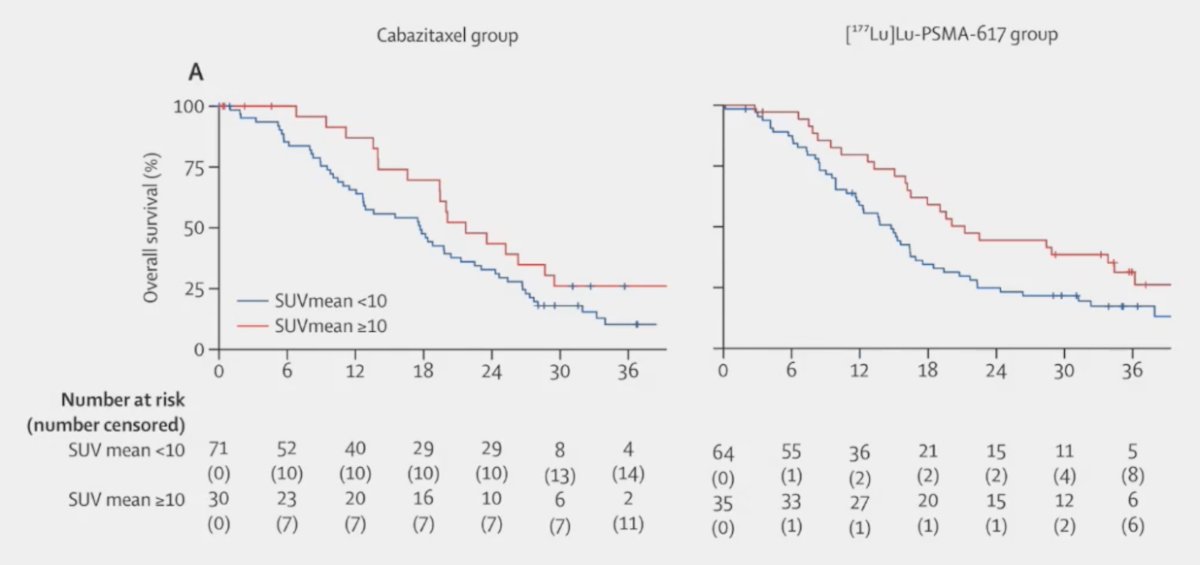
Second, the overall survival was significantly worse for the screen failures compared to the randomized patients. Thus, patients with PSMA PET-negative disease have a poor prognosis: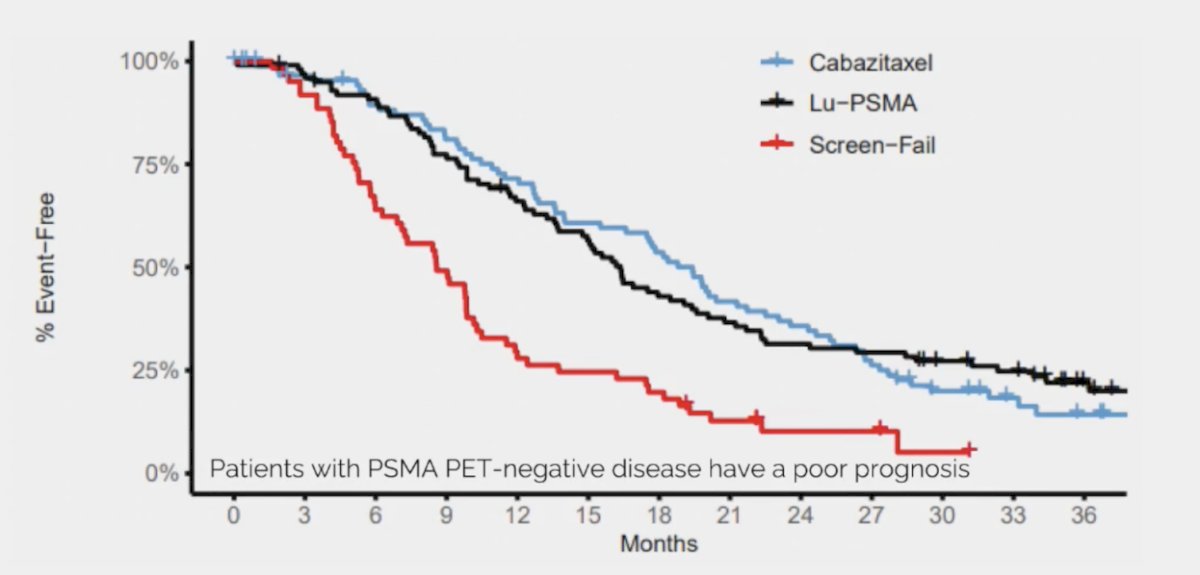
Dr. Smith concluded his presentation discussing the patients who should receive chemotherapy before PSMA radioligand therapy with the following conclusions:
- The optimal sequencing of chemotherapy and PSMA radioligand therapy is undefined
- The cross-trial comparison suggests that 177Lu-PSMA-617 may have a higher PSA response rate than docetaxel for patients treated with mCRPC and prior ARPI
- Cross-trial comparisons suggest that 177Lu-PSMA-617 and cabazitaxel have similar efficacy for patients with mCRPC treated with prior ARPI and docetaxel
- The TheraP study suggests that 177Lu-PSMA-617 has improved PSA response rate and radiographic progression-free survival compared to cabazitaxel, but with no difference in survival
So, according to Dr. Smith, the optimal sequence of chemotherapy and PSMA radioligand therapy in his opinion is as follows:
- Recommend PSMA radioligand therapy before chemotherapy for patients who (i) meet imaging criteria for PSMA radioligand therapy, and (ii) are either medically unsuitable for chemotherapy or express a strong preference to avoid chemotherapy
- Recommend chemotherapy for patients who do not meet the imaging criteria for PSMA radioligand therapy. PSMA PET-negative disease is associated with worse prognosis but is probably not predictive
- For chemotherapy-eligible patients who meet imaging criteria for PSMA radioligand therapy, there are no evidence-based criteria to inform sequencing. Liver metastasis and low SUVmean are prognostic but are probably not predictive biomarkers
Presented by: Matthew R. Smith, MD, PhD, Massachusetts General Hospital Cancer Center, Boston, MA
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
- Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1513-1520.
- Merseburger AS, Attard G, Aström L, et al. Continuous enzalutamide after progression on metastatic castration-resistant prostate cancer treated with docetaxel (PRESIDE): An international, randomized, phase 3b study. Lancet Oncol. 2022 Nov;23(11):1398-1408.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Hofman MS, Emmett L, Sandhu S, et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomized, open-label, phase 2 trial. Lancet Oncol. 2024 Jan;25(1):99-107.
Related Content:


