(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the identification, assessment, and management of side effects of systemic therapies, and a presentation by Dr. Noel Clarke discussing the importance of bone protection.
Epidemiologic data from an English cancer registry study suggests that the 5-year cumulative incidence of skeletal-related events is 1.0% in patients with localized disease, 6.0% in patients with locally advanced disease, and 42.3% in patients with metastatic disease:1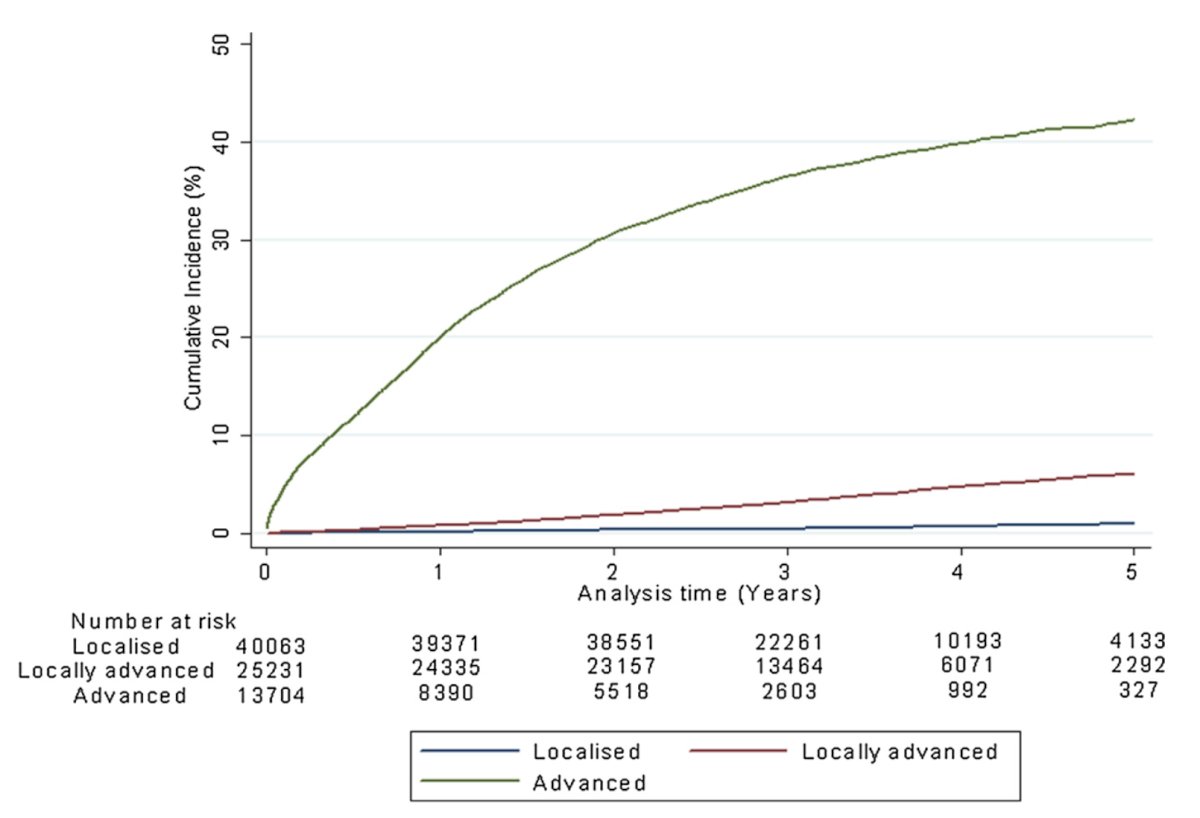
Dr. Clarke notes that FRAX Scoring is not particularly useful for predicting fracture risk in prostate cancer patients. This is primarily because only 25% of participants used in the study that developed FRAX were men, and BMD and FRAX may underestimate fracture risk in men with mHSPC on lifelong ADT. Referencing unpublished work from the STAMPEDE database, Dr. Clarke notes that FRAX may help to stratify fracture risk in M1 patients, but significantly underestimates the actual risk: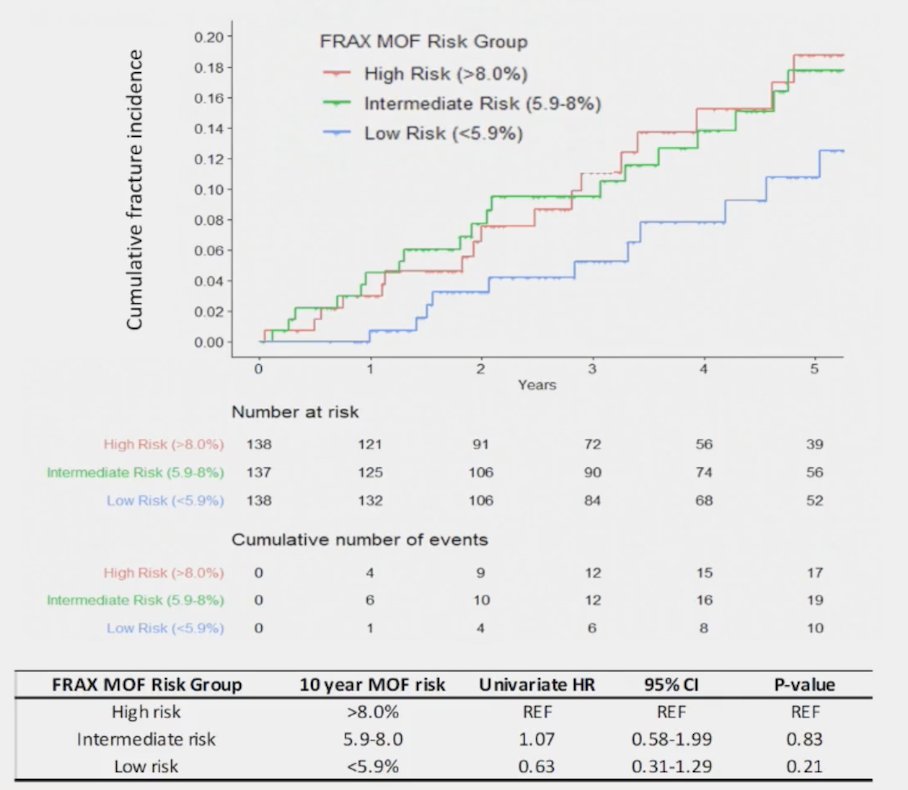
In a secondary analysis of the STAMPEDE phase 3 trials of docetaxel and zoledronic acid using healthcare systems data (manuscript in submission 2024), Dr. Clarke notes that both standard of care + zoledronic acid and standard of care + docetaxel + zoledronic acid decrease the risk of fracture-related hospitalizations in high-risk M1 mHSPC, but with less of an effect in high risk localized prostate cancer (M0):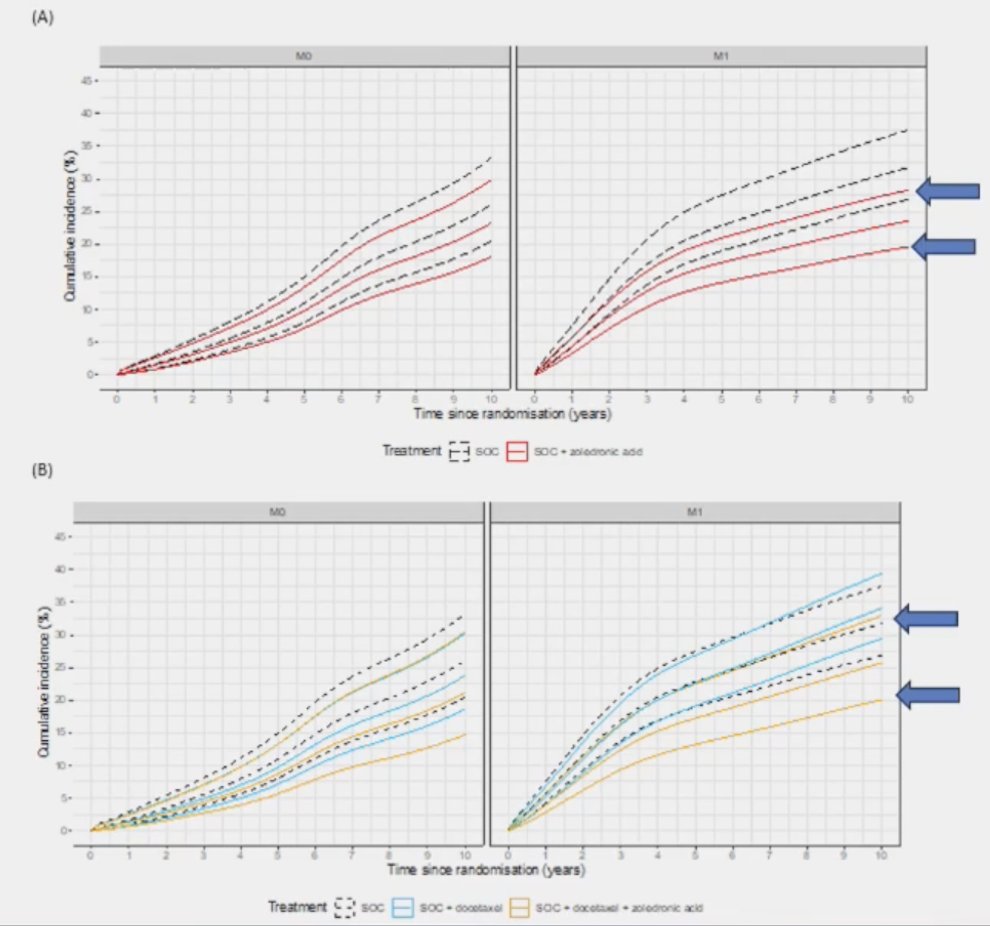
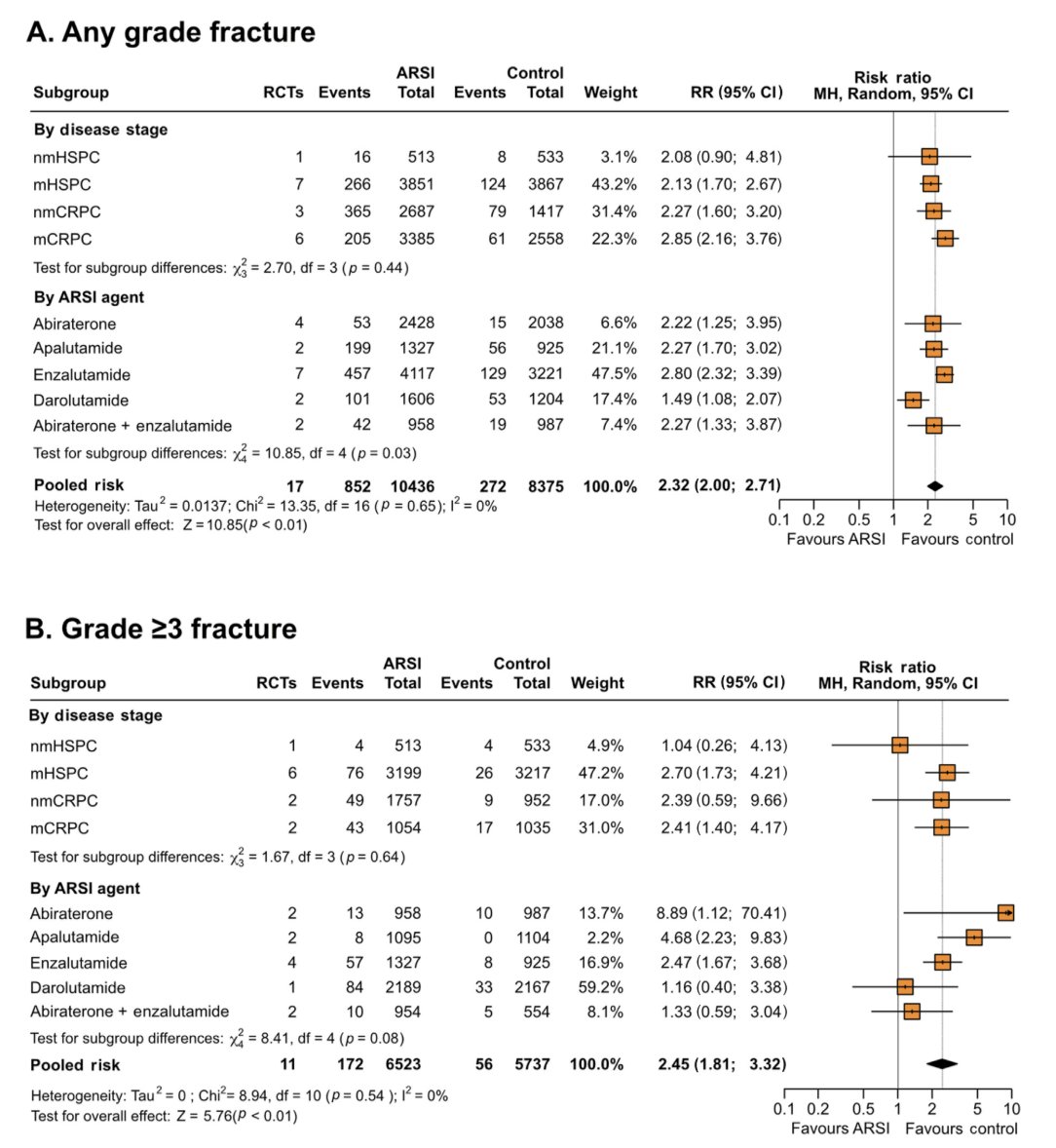
A recently published systematic review assessing the risk of fractures and falls in men with advanced or metastatic prostate cancer receiving ADT and treated with novel androgen receptor signaling inhibitors (ARSIs).2 This pooled analysis demonstrated that ARSIs increased the risk of fractures (RR 2.32, 95% CI 2.00-2.71; p < 0.01) and falls (RR 2.22, 95% CI 1.81-2.72; p < 0.01) compared with control. A subgroup analysis demonstrated an increased risk of both fractures (RR 2.13, 95% CI 1.70-2.67; p < 0.01) and falls (RR 2.19, 95% CI 1.53-3.12; p < 0.0001) in mHSPC patients, and an increased risk of fractures in the nonmetastatic (RR 2.27, 95% CI 1.60-3.20; p < 0.00001) and metastatic castrate-resistant (RR 2.85, 95% CI 2.16-3.76; p < 0.00001) settings. The outcomes of this analysis are as follows:
Dr. Clarke then discussed several outcomes from the study “Fracture-related hospitalizations in de novo advanced or metastatic hormone-sensitive prostate cancer: Secondary analysis of the STAMPEDE abiraterone acetate plus prednisone +/- enzalutamide and M1|RT phase 3 trials using healthcare systems data.” The standard of care versus standard of care + abiraterone acetate + prednisone arm had 99% of eligible trial patients in England successfully linked to HES. This analysis showed a significant reduction in fracture hospitalizations among M1 patients for standard of care + abiraterone acetate + prednisone (HR 0.63, 95% CI 0.49-0.82), but not in M0 patients (HR 0.98, 95% CI 0.72-1.32):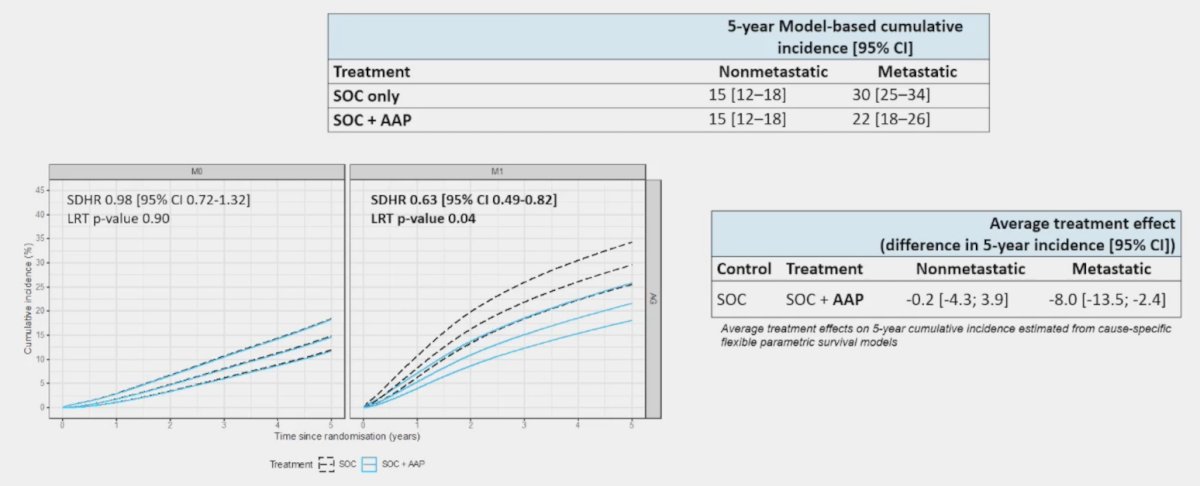
The standard of care versus standard of care + abiraterone acetate + prednisone + enzalutamide arm had 96% of eligible trial patients in England successfully linked to HES. This analysis showed a significant reduction in fracture hospitalizations among M1 patients for the standard of care + abiraterone acetate + prednisone + enzalutamide (HR 0.69, 95% CI 0.54-0.88), but not in M0 patients
(HR 0.76, 95% CI 0.54-1.06):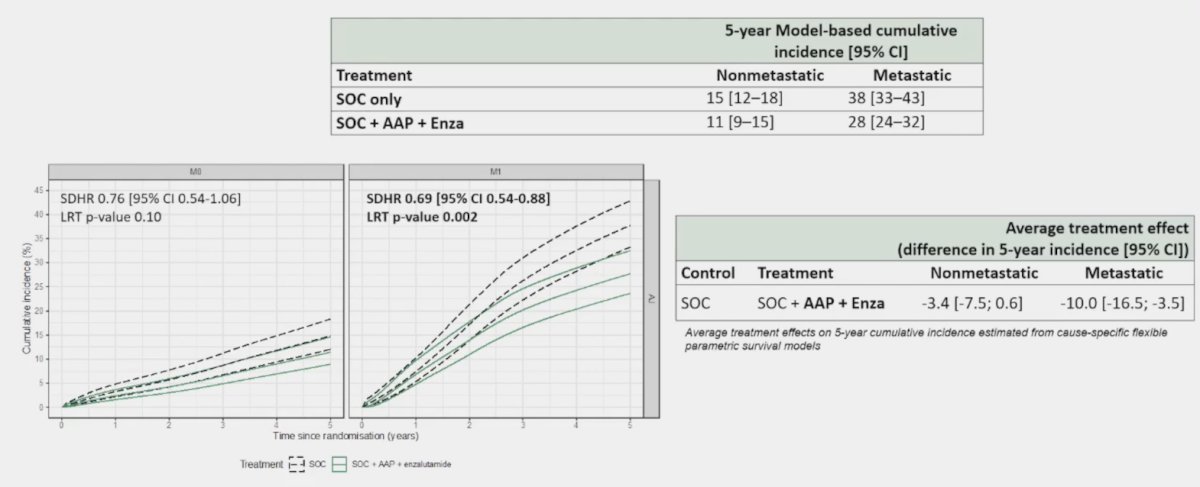
The standard of care versus standard of care + radiotherapy arm had 98% of eligible trial patients in England successfully linked to HES. This analysis did not show a significant reduction in fracture
hospitalizations among M1 patients (HR 0.92, 95% CI 0.79-1.08):
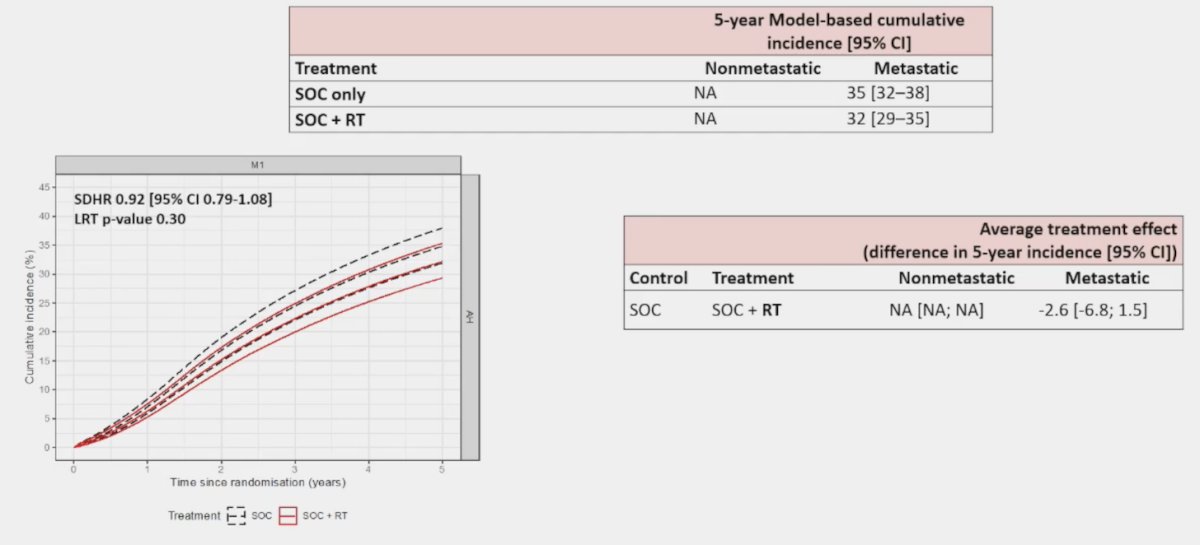
The following forest plot summarizes the aforementioned outcomes: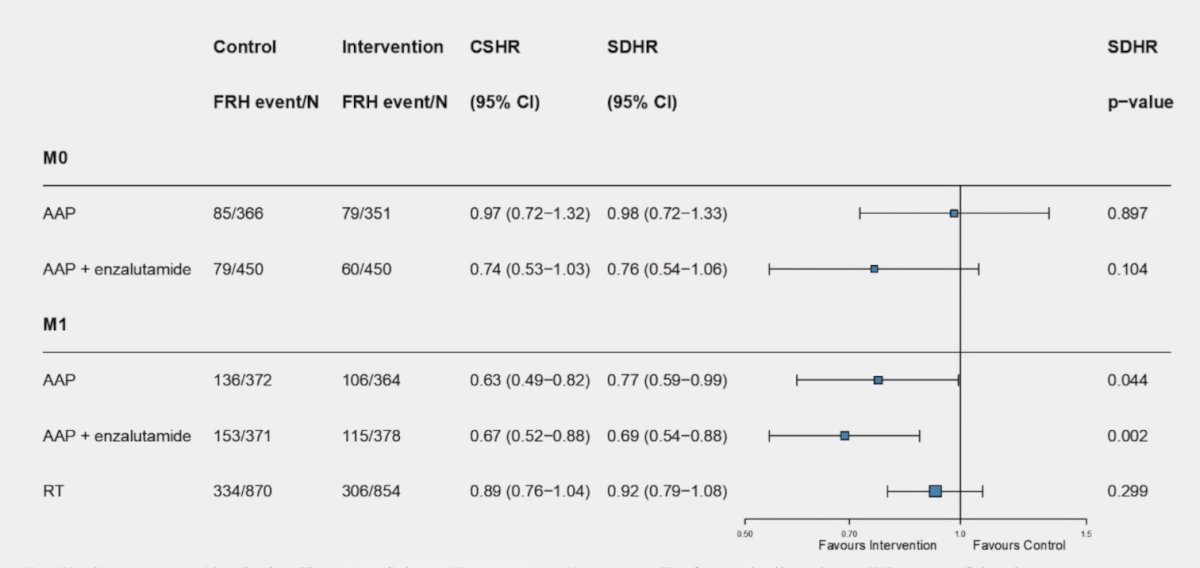
In M0 disease, abiraterone + prednisone +/- enzalutamide there is no evidence of a difference in fracture risk, and in M1 disease, abiraterone + prednisone +/- enzalutamide significantly reduced fracture risk.
Dr. Clarke concluded his presentation discussing the importance of bone protection with the following take-home messages:
- The 5-year cumulative incidence of fracture-related hospitalizations was high in M0 (15%) and M1 (38%) patients treated with ADT only from the abiraterone + prednisone + enzalutamide comparison
- Treatment intensification with abiraterone acetate with prednisone +/- enzalutamide significantly reduces the risk of fractures in patients with mHSPC
- There is no evidence of an effect on fracture risk with the addition of abiraterone acetate with prednisone +/- enzalutamide in non-metastatic patients or in metastatic patients treated with prostate radiotherapy
Presented by: Noel W. Clarke, MBBS, FRCS, ChM, The Christie and Salford Royal Hospitals, Manchester, UK
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
Related content: Bone Health in Advanced Prostate Cancer: Findings from the STAMPEDE Trial and Hospital Episode Statistics - Noel Clarke
References:
- Parry MG, Cowling TE, Sujenthiran A, et al. Identifying skeletal-related events for prostate cancer patients in routinely collected hospital data. Cancer Epidemiol. 2019 Dec;63:101628.
- Jones C, Gray S, Brown M, et al. Risk of fractures and falls in men with advanced or metastatic prostate cancer receiving androgen deprivation therapy and treated with novel androgen receptor signaling inhibitors: A systematic review and meta-analysis of randomized controlled trials. Eur Urol Oncol. 2024 Feb 19 [Epub ahead of print].
Related Content:


