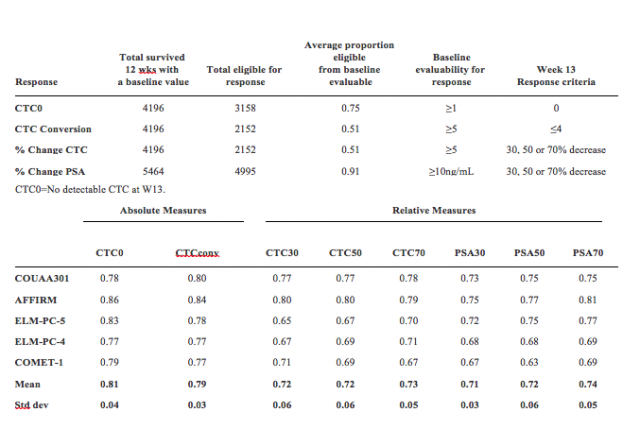
The authors planned to use CTC as a response endpoint and contrast it with PSA. CTCs are tumor cells that shed from the primary tumor and circulate in the blood. CTC number is prognostic for survival at baseline in trials of agents with different mechanisms. CTC number is acquired by CellSearch®, and this value together with PSA values were analyzed in patients who survived at least 13 weeks in these 5 major phase 3 randomized clinical trials: COU-AA-301, AFFIRM, ELM-PC-5, ELM-PC-4 and COMET-1. Patients with missing values at week 13 were considered non-responders. The study results are shown in the table below with CTC0 and CTC conversion producing response measures that were most informative for survival. The discriminatory strength of the response endpoints with respect to overall survival (OS) was estimated using the weighted c-index. To summarize discrimination results for each measure, the mean and the standard deviation based on the weighted c-indices from the 5 studies were computed.
In conclusion, CTC0 and CTC conversion endpoints had the highest discriminatory power for OS relative to the % decline in CTC or PSA endpoints. Dr. Heller therefore suggested the usage of CTC0 as a response endpoint for early phase clinical trials of mCRPC patients.
Presented By: Glenn Heller, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre
Twitter: @GoldbergHanan
at the 2017 ASCO Annual Meeting - June 2 - 6, 2017 – Chicago, Illinois, USA


