Patients enrolled in KEYNOTE-052 were eligible if they:
- Were cisplatin ineligible (ECOG performance status 2, or CrCl ≥30 to <60 mL/min, or grade ≥2 neuropathy/hearing loss, or NYHA Class 3 heart failure)
- Had advanced urothelial carcinoma
- Had received no prior chemotherapy for metastatic disease
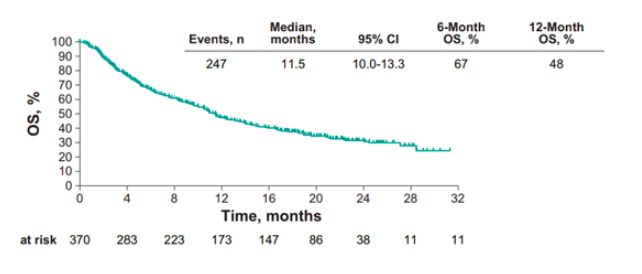
For the updated KEYNOTE-052 analysis, efficacy and safety were assessed in 370 patients over a median follow-up of 11.5 months (range 0.1-31.3 months). The median age was 74 years, including 10.8% of patients ≥85 years of age. There were 42.2% of patients that were ECOG performance status 2, and 85.1% had visceral disease. Confirmed ORR was 28.9% (95%CI 24.3-33.8), including 30 (8.1%) patients with complete response and 77 (20.8%) patients with partial response. The median duration of response was not reached (95%CI, 21.4 months-NR), including 82% who had a response of ≥6 months and 68% who had a response of ≥12 months. The median OS was 11.5 months (95%CI 10.0-13.3) and the 6-and 12-month OS rates were 67% and 48%, respectively.
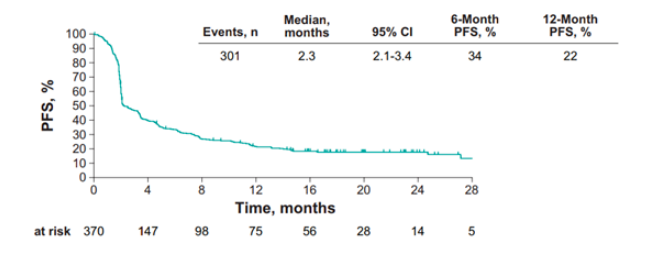
The median PFS was 2.3 months (95%CI 2.1-3.4) and the 6-and 12-month PFS rates were 34% and 22%, respectively.
Among patients with a PD-L1 expression combined positive score of ≥10 (n=110), ORR was 47.3% (95%CI 37.7-57.0) and median OS was 18.5 month (95%CI 12.2 months-NR).
Further subgroup median OS results included:
- Lymph node-only disease (n=51): not reached (95%CI 12.4 months-NR)
- ECOG performance status 0/1 (n=214): 13.1 months (95%CI 11.0-16.8)
- ECOG performance status 2 (n=156): 9.7 months (95%CI 5.7-11.6)
Similar to atezolizumab for advanced urothelial cancer, pembrolizumab appears to have clinically meaningful and durable results (follow-up time more than twice as long as reported in the initial analysis) in a heavily treated and comorbid population. For advanced urothelial carcinoma patients that are cisplatin-ineligible, these results are quite encouraging. Data further support the use of pembrolizumab for patients with urothelial carcinoma who are cisplatin ineligible and those for whom chemotherapy may not be suitable.
Clinical trial information: NCT02335424
Presented By: Jacqueline Vuky, Oregon Health & Science University, Portland, OR
Co-Authors: Arjun V. Balar, Daniel E. Castellano, Peter H. O'Donnell, Petros Grivas, Joaquim Bellmunt, Thomas Powles, Dean F. Bajorin, Noah M. Hahn, Ronald De Wit, Mary Savage, Lei Pang, Tara L. Frenkl, Stephen Michael Keefe, Elizabeth R. Plimack; Oregon Health & Science University, Portland, OR; Perlmutter Cancer Center, NYU Langone Medical Center, New York, NY; Hospital Universitario 12 de Octubre, Madrid, Spain; The University of Chicago Medical Center, Chicago, IL; Cleveland Clinic, Cleveland, OH; Dana-Farber Cancer Institute, Boston, MA; Barts Cancer Institute, Queen Mary University of London, London, United Kingdom; Memorial Sloan Kettering Cancer Center, New York, NY; The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD; Erasmus MC Cancer Institute, Rotterdam, Netherlands; Merck & Co., Inc., Kenilworth, NJ; Fox Chase Cancer Center, Philadelphia, PA
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, Twitter: @zklaassen_md at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA
References:
1. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.


