Atezolizumab is a humanized IgG1 monoclonal antibody targeting PD-L1 and is associated with long-term durable remissions in subsets of patients with metastatic urothelial carcinoma, as reported in the IMvigor210 and 211 studies [3-5]. The ABACUS study investigates the efficacy and safety of neoadjuvant atezolizumab given prior to cystectomy in operable urothelial MIBC.
This single arm, phase II study investigated two cycles of atezolizumab (1200mg every three weeks) prior to radical cystectomy among patients with T2-4N0M0 urothelial carcinoma. Other key eligibility criteria included residual disease post-TURBT and not fit for/rejected cisplatin-based chemotherapy. The co-primary endpoints for the study were pathological complete response occurring in ≥20% of patients, and increase in CD8 count as a biomarker analysis. Secondary endpoints included safety and radiological response to treatment. Cross-sectional imaging was performed at baseline and prior to radical cystectomy which occurred 4 - 8 weeks after starting atezolizumab. Adverse events (AEs) and surgical complications were assessed using CTCAE v4.03 and the Clavien-Dindo classification.
There were 74 patients that received atezolizumab (n=59 received two cycles; n=15 received one cycle). Among these patients, 67 underwent subsequent radical cystectomy. Among the seven patients not receiving surgery was one patient with disease progression (treated with chemotherapy) who were included in the analysis of the primary efficacy endpoint (n=68). The median age of the 68 patients was 71 years (range 53-85), and the baseline pT2 rate was 71%, pT3 was 22%, and pT4 was 7%. The pathologic complete response rates were as follows:
- All patients: 20/68 (29%) – pT0, n=16; pTis, n=4
- PD-L1 positive patients: 10/25 (40%)
- PD-L1 negative patients: 5/31 (16%)
- cT2 patients: 17/48 (35%)
- cT3-T4 patients: 3/20 (15%)
The most common any grade treatment-related AE was fatigue (21%) and most common grade 3/4 AE was transaminitis (4%). Grade 3/4 Clavien-Dindo complications occurred in 10% of patients undergoing radical cystectomy, with no post-operative deaths. There was 1 potential treatment-related death during treatment/perioperative period (cardiovascular disease).
Powles concluded with several important take home points:
- Two cycles of atezolizumab are safe and well tolerated prior to cystectomy in patients ineligible for cisplatin-based chemotherapy
- Pathologic complete responses occurred in 29% of patients which enriched to 40% in PD-L1 expression patients
- Significant progression of the disease appears infrequent with this approach (n=1)
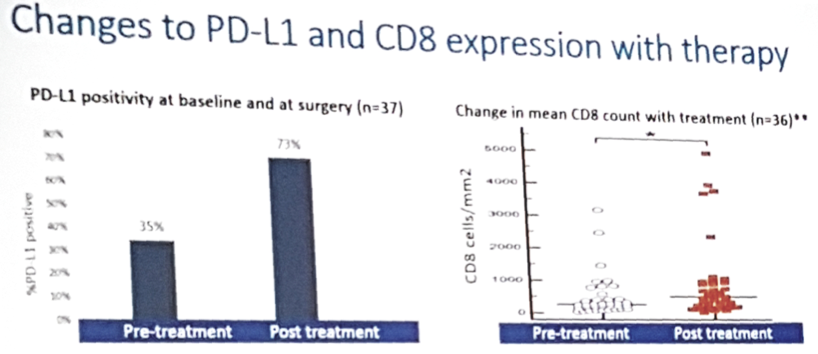
- Sequential biomarker analysis showed an increase in PD-L1 and CD8 expression with atezolizumab
- These preliminary results are promising in this area of unmet need, although long-term follow-up is needed
Clinical trial information: NCT02662309
References:
1. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
2. Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long term results of the BA06 30894 trial. J Clin Oncol 2011;29(16):2171-2177.
3. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909-1920.
4. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017;389(10064):67-76.
5. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomized controlled trial. Lancet 2018;391:748-757.
Presented by: Thomas Powles, MD, Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
Co-Authors: Alejo Rodriguez-Vida, Ignacio Duran, Simon J. Crabb, Michiel Simon Van Der Heijden, Albert Font Pous, Gwenaelle Gravis, Urbano Anido Herranz, Andrew Protheroe, Alain Ravaud, Denis Maillet, Maria Jose Mendez-Vidal, Cristina Suarez, Anja Lorch, Cora N. Sternberg, Mark David Linch, Shah-Jalal Sarker, Aaron Prendergast, Kelly Mousa, Daniel E. Castellano; Hospital Parc Salut del Mar, Barcelona, Spain; Hospital Universitario Marques de Valdecilla, Seville, Spain; Southampton Clinical Trials Unit, University of Southampton, Southampton, United Kingdom; Department of Medical Oncology, The Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands; Institut Català d’Oncologia, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Medical Oncology, Institut Paoli-Calmettes, Marseille, France; Hospital Clínico Universitario de Santiago, Santiago De Compostela, Spain; Churchill Hospital, Oxford, United Kingdom; Department of Medical Oncology, Hôpital Saint-André, University of Bordeaux-CHU, Bordeaux, France; Hosp Lyon SUD, Lyon, France; Reina Sofía University Hospital, Cordoba, Spain; Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Universitat Autònoma de Barcelona, Barcelona, Spain; Heinrich Heine University, Duesseldorf, Germany; San Camillo-Forlanini Hospital, Rome, Italy; Royal Marsden Hospital, Kingston, United Kingdom; Queen Mary University of London, London, United Kingdom; Hospital 12 de Octubre, Madrid, Spain
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, Twitter: @zklaassen_md at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


