It is well known that ADT generates castrate levels of testosterone – at least by the standards available by conventional lab testing. However, evidence has shown that intratumoral androgen levels are still present and that ADT does not suppress it completely. Perhaps lowering the androgen levels even further at the earliest sign of BCR may yield longer, more durable responses?
Considering that recent trial results have demonstrated survival benefit with adding abiraterone (AA) + prednisone (P) to ADT in the de novo metastatic setting, it was inevitable that AA+P would be considering in the BCR setting. Especially since there is evidence that AAP + ADT decreases blood and intratumoral testosterone (T) levels by ≥1 log and in pre-RP trials resulted in greater pathologic responses relative to ADT alone.
In this study, the authors assessed the benefit of AAP in the BCR setting following primary definitive therapy. Post RP ± salvage radiotherapy patients with a rising PSA ≥1.0 ng/ml, doubling time ≤9 months (mo), no metastases (met) on CT/bone scan, and T ≥150 ng/dl were eligible; prior ADT ≤8 mo was allowed. This was a randomized clinical trial with 3 arms: AA 1000 mg + P 5 mg QD (Grp 1), AAP + monthly D (Grp 2), or monthly D (Grp 3) for 8 mo, followed by cessation of treatment.

Prostate cancer trials have been plagued by the slow natural history of the disease. Waiting for overall survival often requires years, and unfortunately, the treatment paradigms are changing much faster than that. Novel endpoints are desperately needed; ideally with shorter time frames. Yet, linking these endpoints with cancer-specific survival and overall survival is also important. In this study, the authors test a new endpoint: undetectable PSA [PSA0] with T recovery [rec], indicating that despite testosterone recovery, prostate cancer recurrence is not evidence. The primary endpt was PSA0 with T > 150 at 18 mo; secondary endpoint was PSA0 at 8 mo.
They treated 120 pts on trial, of which 113 were evaluable for the endpoint at this time.
Patient characteristics:
1. Median age 65
2. 88% White
3. Median PSA 4.36 (of note, slightly higher in Arm 2 – 5.8, slightly lower in Arm 1 – 3.1)
4. 61% had prior salvage XRT
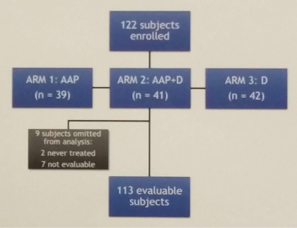
Of these 113 patients, 7 had PSA0 at 18 mo without T rec.
In patients with T recovery, no difference was seen in PSA0 at 8 mo with AAP vs AAP+D (p > 0.99), or AAP vs D (p = 0.11). The PSA0 rate at 8 months was 84%, 86%, and 67% respectively for Groups 1-3.
At 18 months, PSA0 was 5%, 19% and 13%, respectively. Overall, 11.5% of pts achieved the primary endpoint with no difference between groups (Grp 1 vs Grp 3; Grp 2 vs Grp 3 [p = 0.43; p = 0.75]).
Time to PSA progression was best in Arm 2 (AAP+D) and worst in AAP alone. Median time to PSA progression was 37.5, 62.3 and 54.9 months, respectively. P < 0.001.
Interestingly, testosterone recovery was shortest in Group 1 (AAP alone) relative to Grp 3 (p < 0.001). It is unclear if this may lead to earlier recurrence on the long-term.
Treatment related toxicities: The most common AEs were hot flashes (57%), fatigue and injection-site reaction. However, only 3-6 patients (<20%) of patients in each arm had Grade 3 AE – and there were no Grade 4 toxicities.
Long-term follow-up is needed. However, while AAP + D achieved the best results, adding AAP was insufficient to eradicate disease – disease recurrence still occurred.
Presented by: Karen A. Autio, MD
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


