The TOAD (TROG 03.06; NCT00110162) phase 3 randomized trial that showed improved overall survival (OS) and time to clinical progression when immediate androgen deprivation therapy was given, compared with delayed ADT in progressive or relapsed prostate cancer patients after radiotherapy (RT) or prostatectomy + RT.
ELAAT (NCT00439751) was a similar study that failed to reach its accrual goal.
The two investigative teams of these studies planned a combined analysis before ELAAT was activated and now present their results.
This study analyzed patients from the TOAD study (n=261) and ELAAT (n=78 patients). The primary outcome was all cause mortality and secondary outcomes included cause specific mortality (CSM), local progression, distant progression, and CRPC state.
Median follow-up was 5 years in both trials. In the deferred arms of each trial 63% of the TOAD patients received ADT at a median of 1.57 years after randomization; in ELAAT 38% received ADT at a median of 1.65 years after randomization. The mean PSAs at start of ADT in the immediate and deferred arms were 3.52 and 30.2 ng/l for TOAD and 3.98 and 18.1 ng/ml for ELAAT.
The results for CSM, local and distant progression and CRPC status are presented in Figure 1.
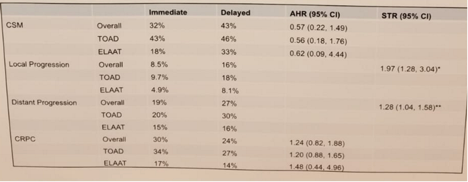 Study results (CSM, progression and CRPC)
Study results (CSM, progression and CRPC)OS is demonstrated in Figure 2, demonstrating no significant difference.
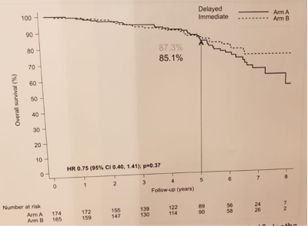 Overall survival
Overall survivalLastly, figure 3 demonstrates the number of death and comparison of local and distant progression.
 Deaths and time to local and distant progression
Deaths and time to local and distant progressionOverall there were 60 deaths (18%) in the combined dataset; with 40% of the deaths in TOAD and 26% if ELAAT.
The authors concluded that no difference in the immediate and deferred arms was seen in OS of these patients. Speaking to the authors, a possible explanation could be that the ELAAT patients were with lower risk of CSM (on average they were lower risk and older) and there was a smaller difference in PSAs between the deferred and immediate arms (mean difference for ELAAT of 14.1 and for TOAD – 26.7 ng/ml).
Presented by: Karen A. Autio, MD
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


