There have been several studies demonstrating that radiotherapy causes an increase in peripheral antitumor immunity. This unique phenomenon is called the abscopal effect. Additionally, radiotherapy has also been shown to induce tumor PD-L1 expression. This can in turn, suppress the anti-tumor immune response. However, inhibition of the PD-1/PD-L1 has been demonstrated to improve anti-tumor immunity by blocking the tumor mediated suppression of cytotoxic T cells.
Nivolumab is a monoclonal antibody directed against the PDL-1. Combining this drug with radiotherapy might have a synergistic effect. The irradiated tumor cell death can enhance antitumor immunity by inducing antigen expression on tumor cells and activate lymphocytes. The combination of SBRT with nivolumab could potentially enhance the antitumor immune response and might improve clinical outcomes.
This study attempted to ascertain whether the anti-tumor immunity of anti-PD1 therapy, in the form of nivolumab, can be enhanced by radiotherapy (SBRT).
To be eligible for the trial, patients had to be older than 18, with an ECOG performance status of 0,1, have MRCC (clear and non-clear cell), demonstrate disease progression after less than 2 prior anti-angiogenic therapies, have a life expectancy of over 12 weeks, and have at least 2 measurable non-brain sites of metastasis, based on RECIST criteria. The trial schema is presented in figure 1.
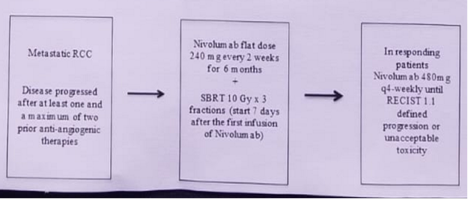
The plan is to enrol 68 patients from 17 centers, assuming a 10% discontinuation rate due to toxicity or non-compliance. The study endpoints included objective response rate (ORR), progression free survival (PFS), overall survival (OS), and safety assessments. Accrual began in July 2017 and so far, 36/68 patients have been accrued.
This is a multicenter, single-arm, phase 2 study in MRCC patients given nivolumab and SBRT in 2nd and 3rd line therapies. All patients will receive nivolumab and SBRT to one non-brain measurable lesion. Nivolumab will be given at a dose of 240 mg in intravenous infusion beginning on day 1 and every 14 days for a duration of 6 months. Then, the dosage will be increased to 480 mg every 4 weeks in responding patients, until unacceptable toxicity or patient death.
According to the trial protocol, no dose reductions or increases will be allowed for nivolumab. All patients will be followed with imaging to assess response, using the RECIST 1.1 criteria with CT scans every 12 weeks.
Presented by: Cristina Masini, Medical Oncology Unit, Clinical Cancer Center, AUSL-IRCCS Reggio Emilia, Reggio Emilia, Italy;
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


