Dr. Heng focused on prognostic criteria (IMDC in particular), first and second line therapy, and the future in this field.
When he sees patients with mRCC, he approaches them as follows:

Patients with better prognosis and limited disease may benefit from metastatectomy. On the other end, patients with advanced metastatic disease may benefit from upfront systemtic therapy. I thought this was a great slide.
Looking at prognostic factors, he obviously focused on the IMDC criteria, though there are other nomograms available.
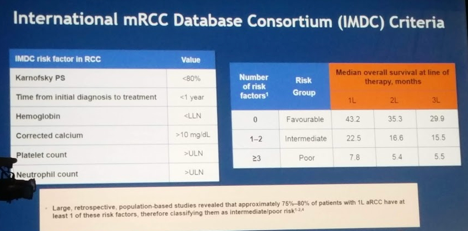
The IMDC criteria takes into account patient factors (PS and time from initial diagnosis of RCC – local or metastatic) and lab values. Simple, yet highly predictive. Risk stratification into favorable, intermediate and poor risk is strongly associated with OS. It has now been validated and is useful in many different stages of RCC management – first-line, second-line, non-clear cell histology, etc.
There are additional prognostic factors described, including location of mets, NLR, etc. However, the question always has to be asked – how much do they add to the current model? If they improve prediction 1-2%, they may not be worth it.
How do we use prognostic factors? For patient counseling, clinical trial stratification (standardization for later comparisons), adjustment methods for retrospective studies, and patient treatment decision making.
He then delved into first and second line therapy for mRCC and recent advances, using the above prognostic factors.
He highlighted CheckMate 214 – phase III trial of Iplimumab/Nivolumab (combined I/O therapy) vs. sunitinib for mRCC. Appeared to have a OS and PFS benefit, particularly in patients who were PD-L1 > 1%. However, based on this, patients with PD-L1 <1% should not be excluded from therapy.
- He did point out that the benefit was primarily in intermediate/poor IMDC risk patients. In the favorable risk group, Ipi/Nivo did worse.
As such, in the first line, Ipi/Nivo should be first line for intermediate/poor risk patients. While PD-L1 status is not predictive of OS, it is for PFS. So, PD-L1 status enriches for patients who may benefit – but should not be used to exclude patients.
In InMotion 151 – Bevacizumab/atezolizumab vs. sunitinib – Bev/atezo appeared to provide PFS benefit in the PD-L1+ patients. Interestingly, in the independent review committee found the benefit wasn’t as strong as initially reported. However, in the PD-L1 negative patients, bev/atezo did have a strong benefit. This is another indicator that PD-L1 status should not be used to exclude patients.
There are numerous phase III I/O combination studies ongoing, so the field will continue to change. Yet, all of these are in clinical trial setting. He emphasized the need for phase IV real-world outcomes comparing these agents – Stukalin et al. (GU ASCO abstract 615) demonstrated that cabo and nivo had similar off-trial effects.
The future: it looks promising! It is likely based on a composite treatment based on biomarker selection. This may include PD-L1 testing, IMDC risk score, and genetic markers (PBRM1, BAP1, etc). Yet, this needs to be further defined.
We are making small steady steps forward towards precision medicine
Presented by: Daniel Heng, MD
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


