Lenvatinib, a novel multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors and other targets, has been approved in combination with everolimus to treat advanced renal cell carcinoma (RCC) after 1 prior VEGF-targeted therapy.(***) Yet, it remains in the second line at this time.
Immune checkpoint inhibitors (ICIs) have been gaining prominence in multiple malignancies, especially in those with high mutational load. In GU oncology, these include bladder and kidney cancer (renal cell carcinoma, RCC). These drugs, despite having a 15-30% objective response rate, have been very exciting as they often display durable response in a small subset of patients. Yet, predicting these responders is not yet feasible.
As a result, combination trials have begun to explore the option of combining traditional therapy and ICIs. One such trial is the phase 1b/2 trial of of lenvatinib and pembrolizumab (anti-PD-L1) in patients (pts) with selected solid tumors, including RCC. Here, the authors present updated results. Final study results are not yet ready.
This is a multicenter, open-label study that included patients with metastatic clear cell RCC. All patients had measurable disease per immune-related RECIST (irRECIST). They all received oral lenvatinib 20 mg daily and pembrolizumab 200 mg IV every 3 weeks. Tumor assessments were performed by study investigators using irRECIST, and were retrospectively reviewed by an independent radiographic review (IRR) per irRECIST and RECIST 1.1.
The primary endpoint of this phase 2 study was objective response rate at 24 weeks (6 months) (ORRWK24) – ORR includes complete response (CR), partial response (PR) and stable disease (SD). Safety was also assessed.
They have enrolled 30 patients in this study, of which 12 (40%) patients had no prior therapy (first-line) and 18 (60%) pts had ≥1 prior anticancer therapy (second-line); 16 (53%) patients had received ≥1 prior VEGF-targeted therapy.
- Median age 62
- 83% Male
- 83% White
- ECOG 0 (67%) or 1 (33%)
- 23% had prior radiotherapy
- 40% PD-L1 positive
At data cutoff of August 1, 2017, median follow-up for progression-free survival (PFS) was 13.8 mos (95% CI, 11.9–15.7).
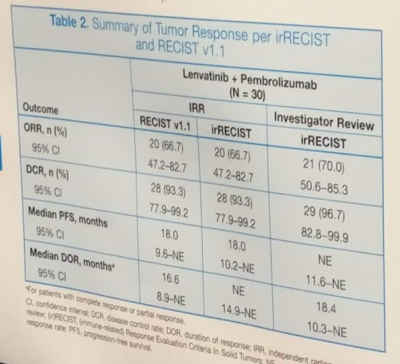
In terms of outcomes, ORRWK24 was 63.3% (95% CI, 43.9–80.1) by investigator review per irRECIST. ORR by IRR using RECIST 1.1 was 66.7% (95% CI, 47.2–82.7) and median PFS was 18.0 mos (95% CI, 9.6–NE). Efficacy outcomes are summarized in the table below:
When stratified by prior treatment status or PD-L1 status, responses were similar (they provided swimmer’s plots to demonstrate this).
Treatment exposure:
Median duration of treatment for levantinib: 15.5 months
Median # of cycles received of pembrolizumab: 21.5
The combination was poorly tolerated, however. Grade 3 or 4 adverse events (AEs) occurred in 73.3% of patients - the most common AEs were diarrhea (83%), fatigue (70%), hypothyroidism (67%), stomatitis (63%), and nausea (60%). Grade 3/4 AE’s included lipase elevation primarily (7 patients), hypertension (4 patients), proteinuria (3 patients) and others (1-2 patients). Despite this, only 26.7% discontinued treatment due to AE. At data cutoff, 2 patients had died – both considered non-treatment related.
As the authors report it, the combination regimen had “manageable AEs, and no new safety signals.” I agree that the oncologic outcomes appeared promising. Accordingly, a phase 3 trial of lenvatinib + pembrolizumab and lenvatinib + everolimus versus sunitinib for the first-line treatment of advanced RCC is underway (NCT02811861). This could be an exciting new first-line or salvage option for patients!
Presented by: Chung-Han Lee, MD
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


