As the moderator, Dr. Grivas kicked off the session by providing the framework for the remainder of the talks. He began by looking at the current utilization and indication for immune checkpoint inhibitors in the management of urothelial carcinoma. First, ICI’s were approved in the second-line setting for metastatic urothelial carcinoma (mUC) – and there are currently 5 agents approved in this space.

Importantly, they all have ~15-20% objective response rate, median PFS ~1-2 months and median overall survival (OS) ~6-18 months. This naturally means that 80-85% are non-responders. And in these patients, there are no other established, effective therapies.
In the front line cisplatin-ineligible setting, there are 2 agents approved – pembrolizumab and atezolizumab, and their data is summarized below:
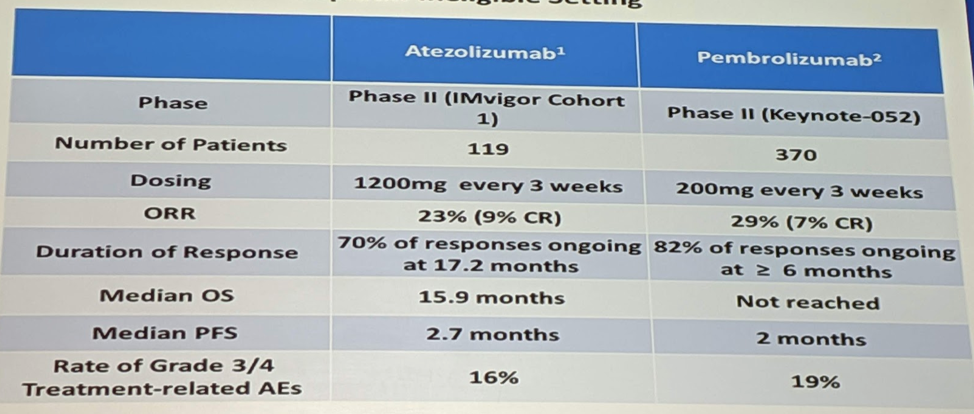
Slightly better objective response rate (ORR) (~20-30%), but similar median progression-free survival (PFS) and OS as in the second line setting.
So, looking forward to rational combinations with current immunotherapies, there are a few considerations he asked:
- Non-overlapping compartments – don’t duplicate mechanism of action
- Primer and effector combination
- Distinct target immune populations
- Avoiding synergistic toxicity (>additive toxicity)
- Optimal timing – is sequencing better? Or a combination?
Work by Terence Friedlander, MD, and his UCSF Urology and Medical Oncology colleagues, in the localized bladder cancer setting, may shed significant light on this. In their study, they are treating patients with atezolizumab in the neoadjuvant setting prior to cystectomy, but are collecting/analyzing significant quantities of data from the patient and specimen. This will help elucidate the local response to ICI, which is lacking currently.
Dr. Grivas briefly reviewed that there are a few predictive biomarkers of response to anti-PD-L1 therapy, including evidence of inflammation (increased PD-L1, increased CD8+ eff/IFN signature) and increased neoantigen/tumor mutational burden (TMB). In addition, work on the molecular subtyping of urothelial cancer has further suggested specific subtypes that are responsive to ICI – Lund genomically unstable and TCGA luminal type II. Work by Galsy et al. (ESMO 2017) has also identified infiltrating T cell abundance (ITA) is associated with EMT and lower OS.
Many of the prior RCTs have provided important biomarker information, including work that Dr. Grivas himself has presented (GU ASCO 2019) from Keynote 052. He noted that higher 18-gene T-cell inflamed GEP score was associated with improved PFS and lower stromal / TGF-beta / EMT score is associated with favorable PFS.
Beyond predictors to help better select current ICI’s, there are numerous other targets for ICI’s on the horizon – it seems new targets are being discovered and agents are being developed to target them quickly. These include CTLA-4, LAG3, TIGIT, TIM3, etc. There are also other immunotherapies targeting IDO, antibody-drug conjugates (ADCs), etc that one of the later speakers will elaborate on.
Another area of interest is personalized vaccines, which a later speaker addressed in greater detail. The concept here is the introduction of either a protein or RNA of a neoantigen derived from a patient’s own tumor, which is then developed and then reintroduced in larger quantities to prime and active the immune system in conjunction with ICIs. This has been developed as a proof of concept in melanoma already and there is work to develop this for bladder cancer. As urothelial carcinoma, like melanoma, has a high tumor mutational burden, there is hope.
Next, one of the later speakers will address the role of combination with chemotherapeutic agents. This includes already established agents such as gemcitabine and cisplatin, but also other agents such as oxaliplatin (more immunogenic than cisplatin). Specific agents may have the potential for synergy with ICIs.
Radiotherapy has already known to have a synergistic effect with chemotherapy. But, radiotherapy is also thought to have a synergistic effect with ICI’s, as it causes neoantigen release and exposure, primes the system, and has the prospect of an abscopal effect. The question becomes how to deliver it and in what clinical context?
Targeted therapies are being developed rapidly as our genomic understanding of urothelial carcinoma rapidly increases. Novel agents that target specific mutations are being developed and tested, often in combination with established therapies. Foremost at this time are the FGFR3 targeting agents, but others are upcoming (HER2, etc.). One of the later speakers will review these.
He summarized these various options below:
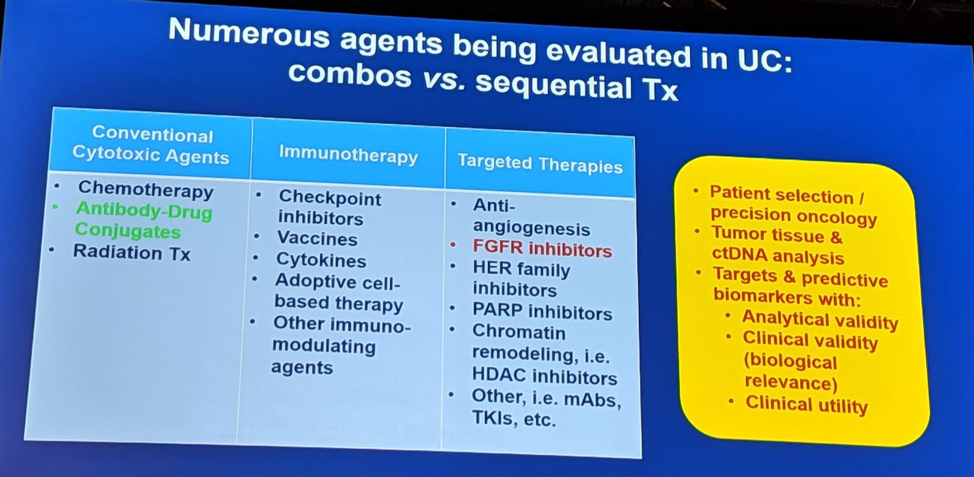
The remaining 3 speakers delved into each of these above topics in more detail.
Presented by: Petros Grivas MD, PhD, Medical Oncologist, Seattle Cancer Care Alliance, Clinical Director, Genitourinary Cancers Program, University of Washington Medicine, Associate Professor of the Department of Medicine, Division of Oncology, University of Washington School of Medicine, Seattle, WA
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Grivas P, Drakaki A, Friedlander TW, Sonpavde G. Conceptual Framework for Therapeutic Development Beyond Anti-PD-1/PD-L1 in Urothelial Cancer. Am Soc Clin Oncol Educ Book. 2019 Jan;39:284-300. doi: 10.1200/EDBK_237449. Epub 2019 May 17. PMID: 31099684


