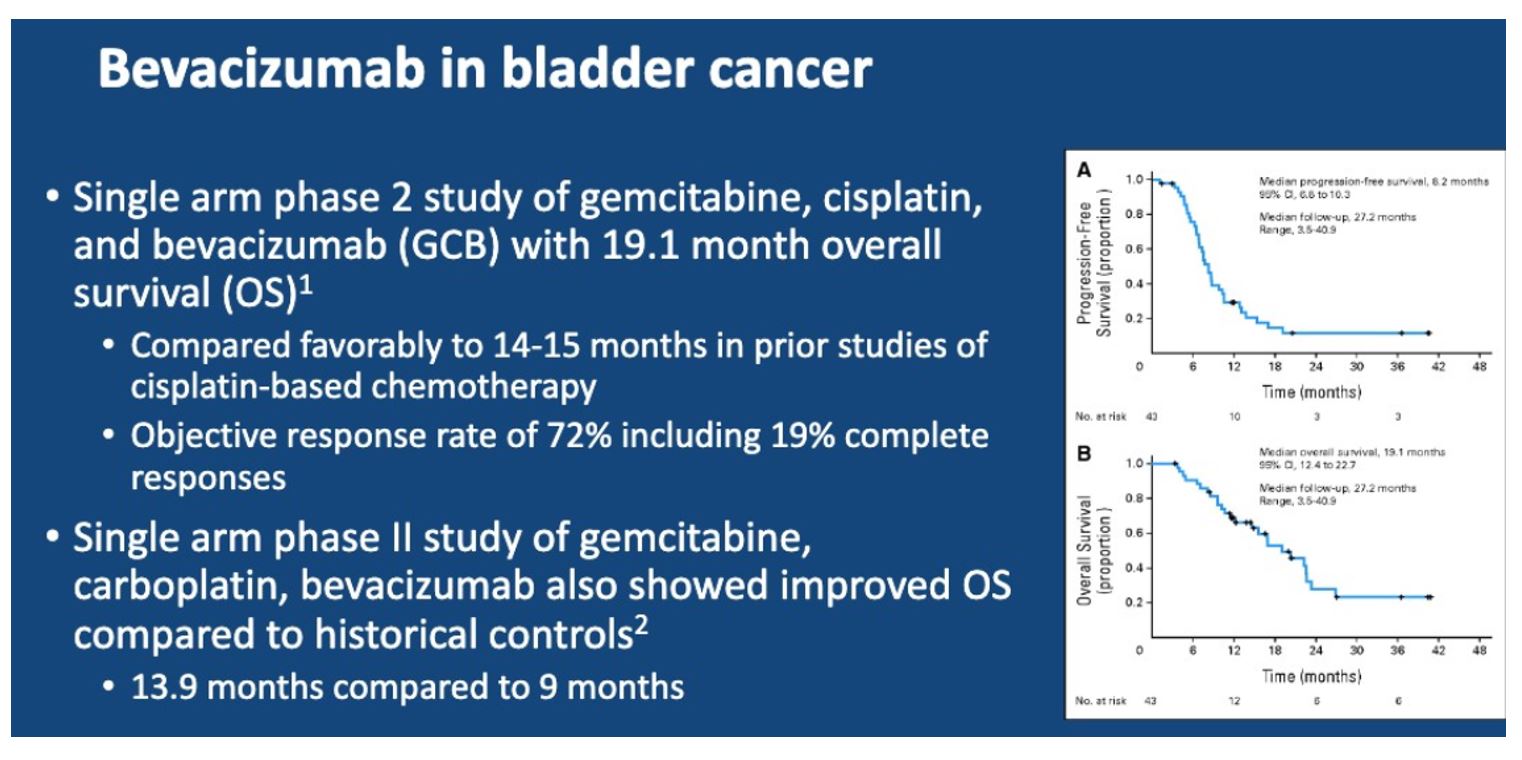
These positive results have led to an additional evaluation of bevacizumab to gem/cis. Here, the authors present the results of a randomized, double-blind, placebo-controlled phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma.
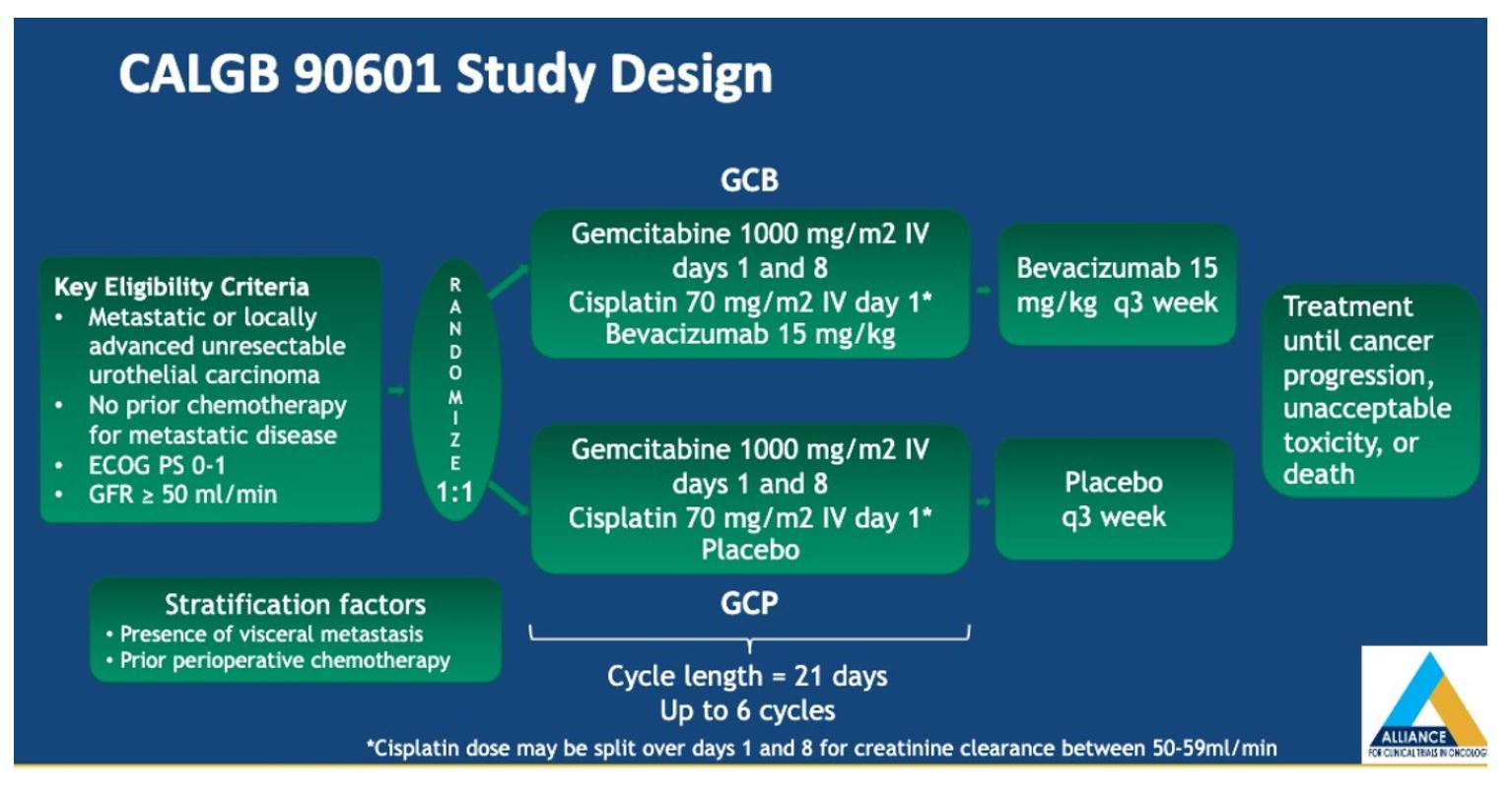

To ensure balance in the two arms, patients were also stratified based on the presence of visceral metastases and prior chemotherapy. The primary endpoint was overall survival, as defined from the time of randomization to death or last follow up. Secondary endpoints included objective response rate (ORR), progression-free survival (PFS), and ≥grade 3 toxicity.
In terms of overall survival, there was no difference between patients treated with GCB vs GCP (14.5 months vs 14.3 months, HR of 0.87 (95%CI 0.72-1.06, p=0.17).

Progression free survival (PFS) was improved with GCB (0.77 (95%CI 0.63-0.93, p=0.0074), with an absolute benefit of one month, which per the author was not clinically significant.

In terms of grade 3 or greater AEs, patients with GCB had 83.5% AEs compared with 80.7% with GCP.

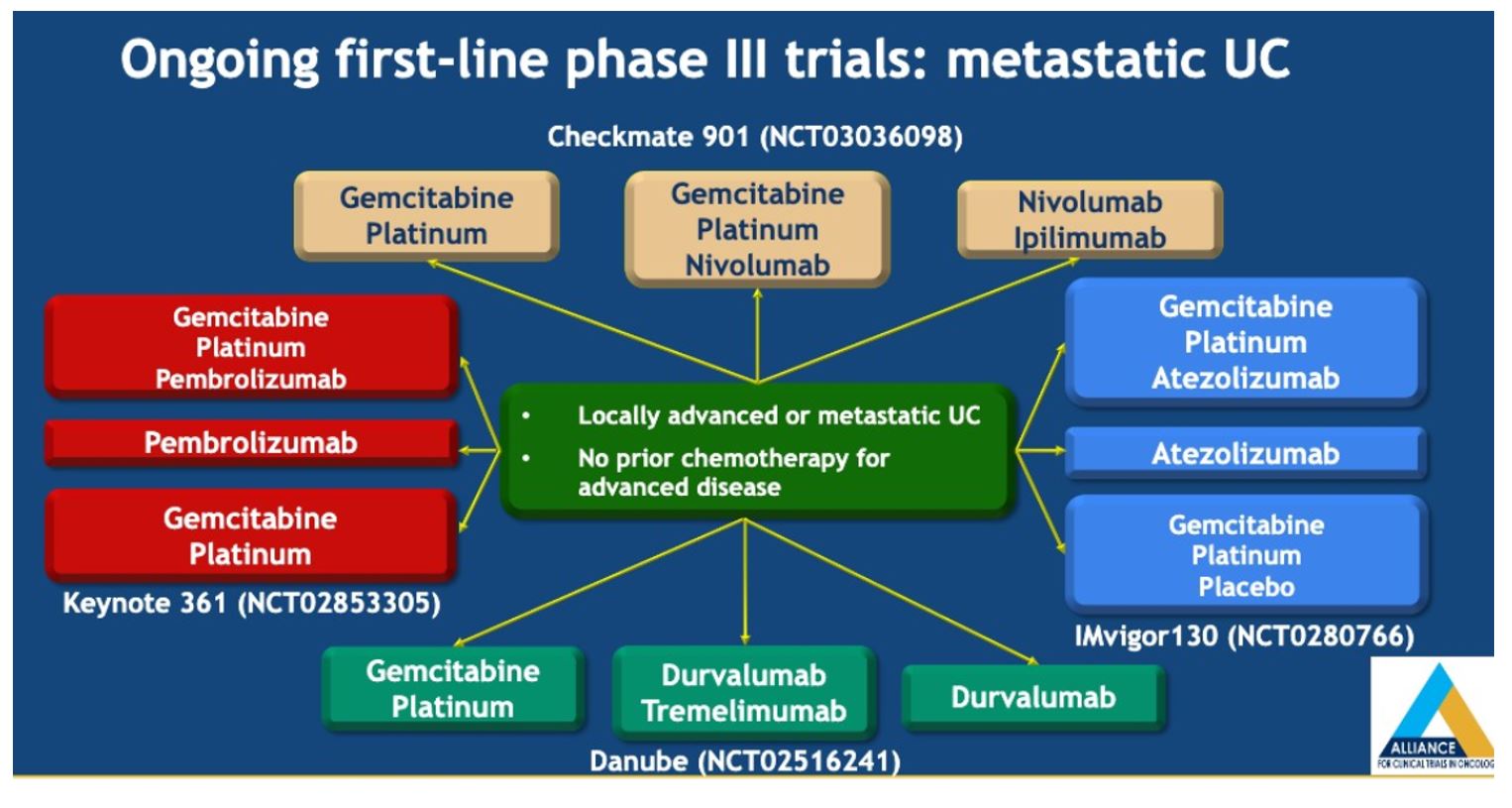
There was no overall survival benefit with the addition of bevacizumab to gemcitabine and cisplatin for patients with metastatic UC who are cisplatin eligible. Cisplatin-based chemotherapies remain standard of care for cisplatin eligible patients with metastatic urothelial carcinoma (mUC) and combination studies are important for improving our front line options. Unfortunately, chemotherapy alone is unlikely to result in durable responses for the majority of patients and a combination approach with immunotherapy may be of benefit -this is being examined in NCT03093922, a randomized phase II study testing two different schedules of atezolizumab combined with gemcitabine and cisplatin chemotherapy (GC) in patients with mUC who are eligible for cisplatin. A number of other ongoing phase III trials are ongoing as shown below.
Presented by: Jonathan E. Rosenberg, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. Journal of clinical oncology 2005;23:4602-8.
- Maase Hvd, Hansen SW, Roberts JT, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. Journal of Clinical Oncology 2000;18:3068-77.
- Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. Journal of Clinical Oncology 2011;29:1525-30.


