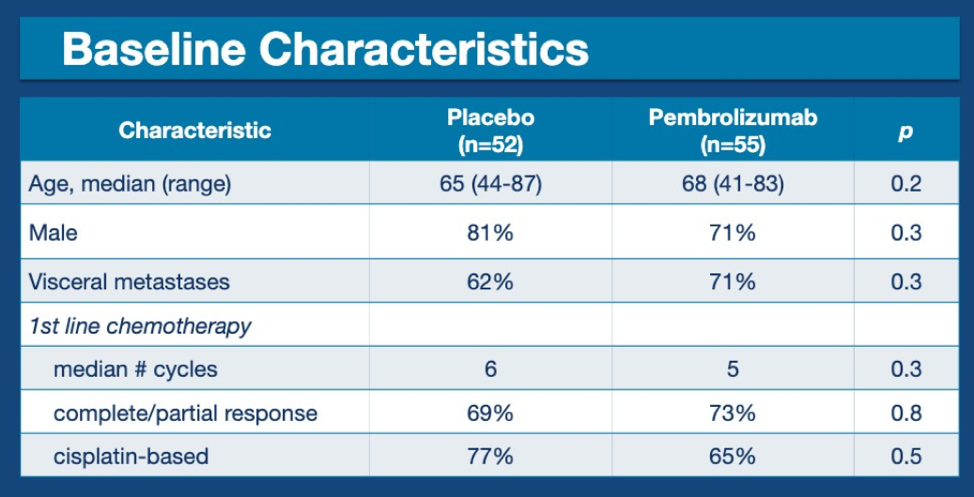
This abstract provides data on 107 patients who were randomly assigned to maintenance placebo or pembrolizumab after receiving platinum-based chemotherapy for mUC. Baseline characteristics are shown below.
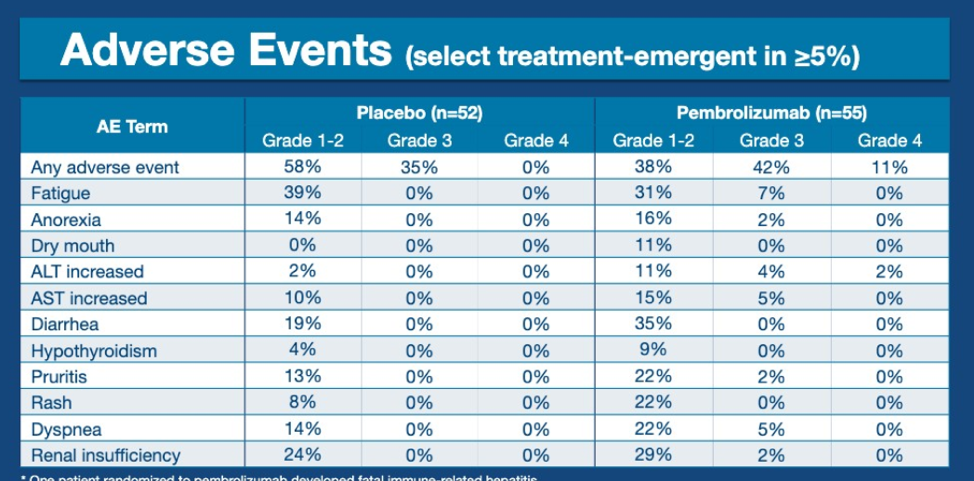
Adverse events were consistent with prior studies of pembrolizumab but this is one of the first studies to show pembrolizumab compared with placebo, both as single agents. There was numerically increased Grade 3/Grade 4 toxicity with pembrolizumab, in particular, fatigue and elevated transaminases. 35% of patients on pembrolizumab had grade 1-2 diarrhea compared with 19% of patients on placebo.
Presented by: Matt D. Galsky, MD, FASCO, Mount Sinai Hospital, New York, NY
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Maase Hvd, Hansen SW, Roberts JT, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. Journal of Clinical Oncology 2000;18:3068-77.
- Loehrer PJ, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. Journal of Clinical Oncology 1992;10:1066-73.
- Grivas PD, Daignault S, Tagawa ST, et al. Double‐blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer 2014;120:692-701.
- Powles T, Huddart RA, Elliott T, et al. A phase II/III, double-blind, randomized trial comparing maintenance lapatinib versus placebo after first-line chemotherapy in HER1/2 positive metastatic bladder cancer patients. American Society of Clinical Oncology; 2015.
- Font A, Perez-Valderrama B, Virizuela JA, et al. Randomized, placebo-controlled phase II trial (MAJA): Efficacy results of maintenance vinflunine after cisplatin chemotherapy (CT) in patients with advanced urothelial carcinoma (UC)—SOGUG 2011-02. American Society of Clinical Oncology; 2016.


