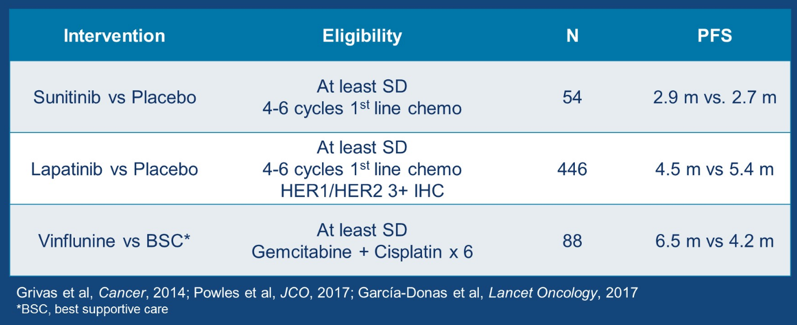
“Switch maintenance” therapy includes a primary treatment followed by maintenance with another treatment modality. There is precedent for switch maintenance therapy in metastatic urothelial cancer (mUC), with three studies assessing progression free survival (PFS) outcomes.
Matt D. Galsky, MD, and colleagues presented results of their phase II assessing maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer.
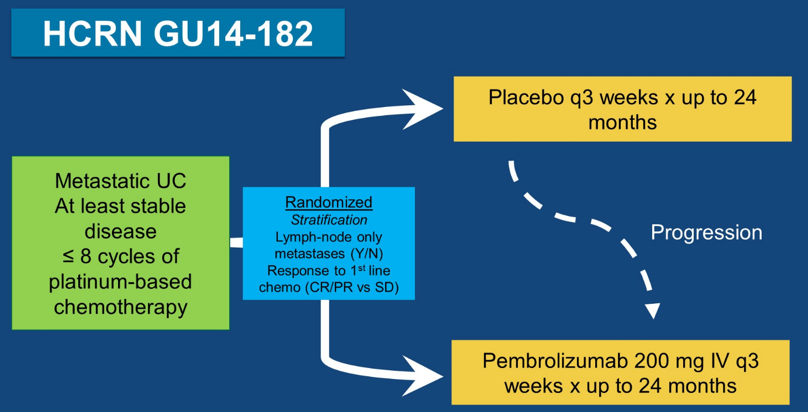
HCRN GU14-182 was an investigator lead study enrolling patients with metastatic urothelial cancer achieving at least stable disease after up to 8 cycles of first-line platinum-based chemotherapy. Patients were randomized 1:1 to pembrolizumab 200 mg IV every three weeks versus placebo for up to 24 months; patients that progressed on placebo could cross over to pembrolizumab. Randomization was stratified based on pre-chemotherapy visceral metastases (yes vs no) and response to first-line chemotherapy (complete response/partial response vs stable disease). The primary objective was to determine the PFS as per irRECIST among patients treated with pembrolizumab versus placebo. The full trial schema is as follows:
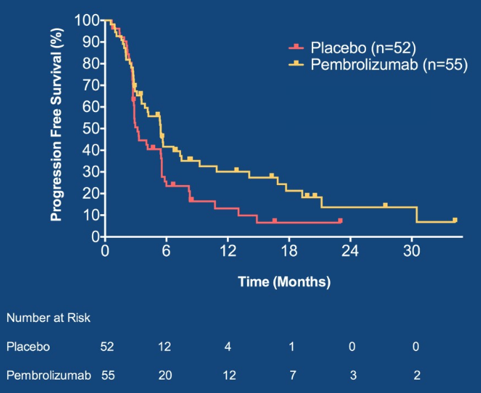
Between December 2015 and November 2018, 107 patients were randomized to placebo (n=52) versus pembrolizumab (n=55). The baseline patient characteristics were well balanced and including ~2/3 of patients with visceral metastases, median 6 cycles of first-line chemotherapy, 2/3 of patients achieving a complete or partial response, and ~2/3 of patients receiving cisplatin-based chemotherapy. Excluding patients with baseline complete responses, the objective response rate was 12% on placebo and 22% on pembrolizumab. Grade 3-4 treatment related adverse events occurred in 48% of patients on placebo and 56% on pembrolizumab. At a median follow-up of 14.7 months, 41 patients died and 26/52 patients randomized to placebo crossed over to pembrolizumab. PFS was significantly longer in patients randomized to pembrolizumab vs. placebo (HR 0.64, 0.41-0.98) Maximum Efficiency Robust Test p=0.036; log-rank p = 0.038):
Dr. Galsky concluded his presentation of HCRN GU14-182 with several take-home messages:
- Switch maintenance pembrolizumab therapy significantly delays disease progression in patients with metastatic urothelial carcinoma
- The adverse event profile is consistent with other treatment settings
- Of note, PFS and OS in PD-L1 positive patients will be reported in the near future
- The role of switch-maintenance PD-1 blockade will be refined by ongoing phase III studies
Presented by: Matt D. Galsky, MD, Department of Medicine, Icahn School of Medicine at Mount Sinai, Tisch Cancer Institute, New York, NY
Co-Authors: Sumanta K. Pal, Amir Mortazavi, Matthew I. Milowsky, Saby George, Sumati Gupta, Mark T. Fleming, Long H. Dang, Daniel M. Geynisman, Radhika Walling, Robert S. Alter, Erwin L. Robin, Jue Wang, Shilpa Gupta, David D. Chism, Joel Picus, George Philips, David I. Quinn, Noah M. Hahn, Menggang Yu; City of Hope National Medical Center, Duarte, CA; Arthur G. James Cancer Hospital, Ohio State University Wexner Medical Center, Columbus, OH; University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; Roswell Park Cancer Institute, Buffalo, NY; Huntsman Cancer Institute-University of Utah Health Care, Salt Lake City, UT; Virginia Oncology Associates, US Oncology Research, Norfolk, VA; Ochsner Medical Center, Baton Rouge, LA; Fox Chase Cancer Center, Philadelphia, PA; Community Cancer Center, Indianapolis, IN; John Theurer Cancer Center at Hackensack University Medical Center, Hackensack, NJ; Community Hospital, Munster, IN; University of Arizona Cancer Center at Dignity Health St. Joseph's Hospital and Medical Center, Phoenix, AZ; Department of Medicine, Masonic Cancer Center, University of Minnesota, Minneapolis, MN; Vanderbilt University Medical Center, Nashville, TN; Washington University in St. Louis School of Medicine, St. Louis, MO; Georgetown Univ Hosp, Washington, DC; USC Norris Comprehensive Cancer Center, Los Angeles, CA; Johns Hopkins University School of Medicine, Baltimore, MD; University of Wisconsin Department of Biostatistics and Medical Informatics, Madison, WI
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA


