Dr. Necchi started his presentation with a figure depicting the clinical states model of bladder cancer:

The CALGB 90601 study was a phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. This study showed no benefit for adding bevacizumab, with a median overall survival (OS) of 14.5 months (95% CI 13.5-16.2) for patients treated with GCB and 14.3 months (95% CI 12.1-16.2) for patients treated with GCP with an HR of 0.87 (95% CI 0.72-1.06). Looking more closely at the two preceding phase II trials assessing bevacizumab in metastatic urothelial carcinoma1, 2, Dr. Necchi suggests that the negative findings in this phase III trial are perhaps not that surprising: in cisplatin eligible patients, the ORR was 72%, median progression-free survival (PFS) of 8.2 months, and median OS of 19.1 months; in cisplatin-ineligible patients, the objective response rate (ORR) was only 49%, with a short median PFS of 6.5 months, and median OS of 13.9 months. This, as Dr. Rosenberg also mentioned, highlights the need to perform phase III trials regardless of phase II study findings. But perhaps there is a subset of patients that may benefit from bevacizumab? Stromal-infiltrated tumors have high angiogenesis signature scores and may benefit from bevacizumab’s anti-angiogenesis mechanism.
The HCRN GU14-182 study assessed maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. This study found that after excluding patients with baseline complete responses, the objective response rate was 12% on placebo and 22% on pembrolizumab. PFS was significantly longer in patients randomized to pembrolizumab vs. placebo (HR 0.64, 0.41-0.98), and the 18-month restricted mean progression-free survival time was 5.6 months with placebo and 8.2 months with pembrolizumab (p=0.023). Dr. Necchi notes that this is an important trial, but states that it would have been nice to know what type of platinum chemotherapy was used. Furthermore, only 107 patients were randomized between December 2015 and November 2018, suggesting that it was challenging to recruit to this study. Finally, because the proportional hazard assumption is not valid with immunotherapy, it was pertinent for the authors to use different statistical methods (restricted mean progression-free survival time), which they did. Dr. Necchi notes that phase III trials enrolling into first-line immunotherapy trials will be crucial. If positive, chemotherapy-immunotherapy combination therapy will be better than maintenance immunotherapy and will be the standard of care. If negative, is there a role for chemotherapy-immunotherapy sequencing (pending biomarker data)?
The EV-201 study is a pivotal phase II study assessing enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors. This study found that enfortumab vedotin is the first novel therapeutic to demonstrate substantial clinical activity in patients who progressed after platinum chemotherapy and a PD-1/L1 inhibitor, with a 44% response rate (12% complete response) and 7.6-month median duration of response. Furthermore, responses were observed across all subgroups and irrespective of response to prior PD-1/L1 inhibitor or presence of liver metastases, with a manageable safety profile. Dr. Necchi notes that the median number of prior systemic therapies was 3, however, the range was 1-6; thus, do we know if the enfortumab vedotin benefit is independent of previous immunotherapy exposure? Additionally, does time from prior therapy make a difference? Objective response data is very promising according to Dr. Necchi, but these findings need to be further assessed in the phase 3 setting. The following slide summaries key data in the salvage setting:
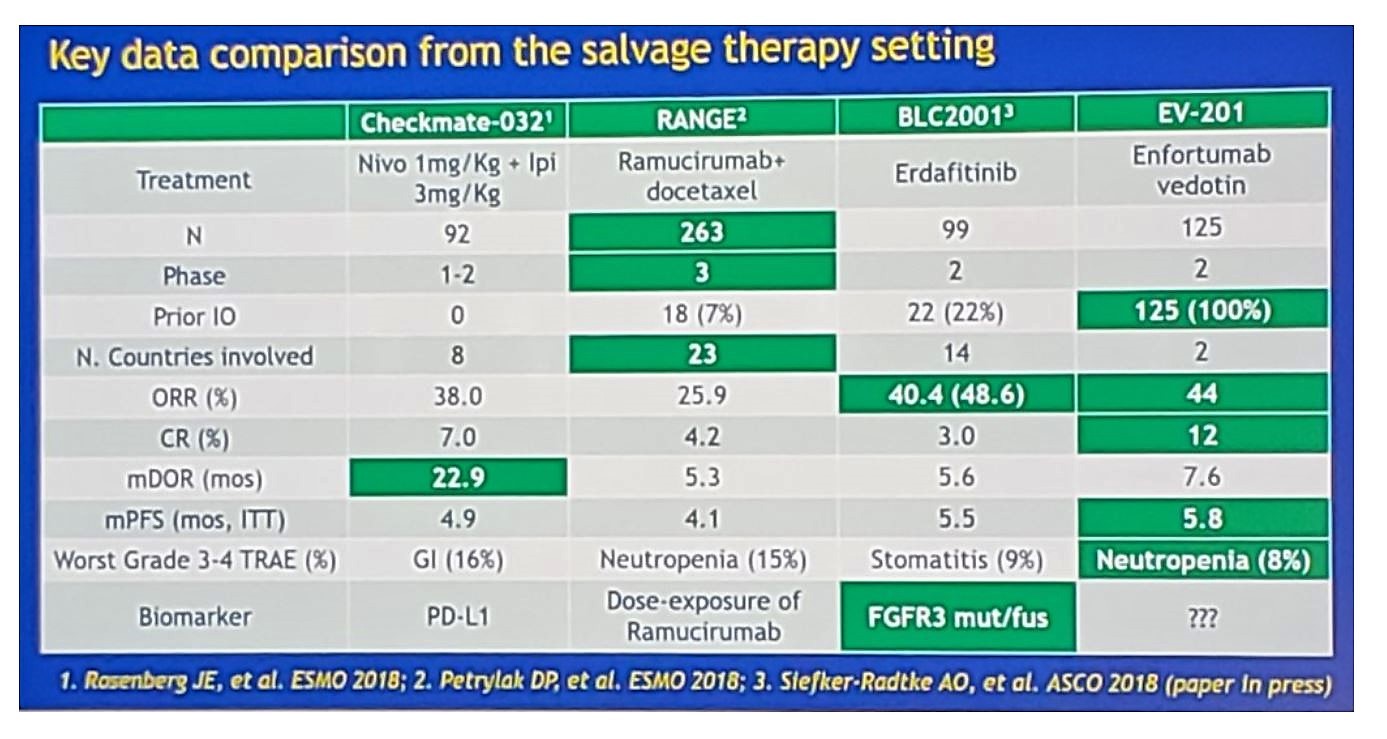
Dr. Necchi concluded his discussion asking the question “How should we navigate through the clinical states?”
- Biomarker data are urgently needed from the presented studies
- Synergy of therapy combinations or sequences needs to be proven
- After immunotherapy failure, ADCs (ie. enfortumab vedotin) are best positioned as next-in-line standard of care
- Outcome prediction tools including biomarker data are necessary to orient patient counselling/inform therapeutic strategies
Presented by: Andrea Necchi, MD, Fondazione IRCC Istituto Nazionale dei Tumori, Milan, Italy
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol 2011;29(12):1525-1530.
- Balar AV, Apolo AB, Ostrovnaya, et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 2013;31(6);724-730.


