The combination of nivolumab and ipilimumab has demonstrated safety and efficacy in urothelial carcinoma and other malignancies, including GU malignancies (renal cell carcinoma). To help address the lack of data in BCVH patients, the authors in this multicenter, single arm, multi-cohort phase II trial evaluated the efficacy of nivolumab and ipilimumab in patients with BCVH and other advanced rare genitourinary cancers.
At ASCO 2019, they reported the preliminary results of the fully accrued BCVH cohort. Eligible patients had metastatic BCVH, ECOG performance status of 0-2 and were either untreated or had received any number of lines of prior therapy - excluding prior immunotherapy. Patients underwent a baseline biopsy and blood collection for correlative studies and received treatment with nivolumab 3 mg/kg and ipilimumab 1 mg/kg intravenously every 3 weeks for 4 cycles with continued maintenance of nivolumab 480 mg IV every 4 weeks. The primary endpoint was overall response rate (ORR) by RECIST 1.1. The full protocol can be seen below:

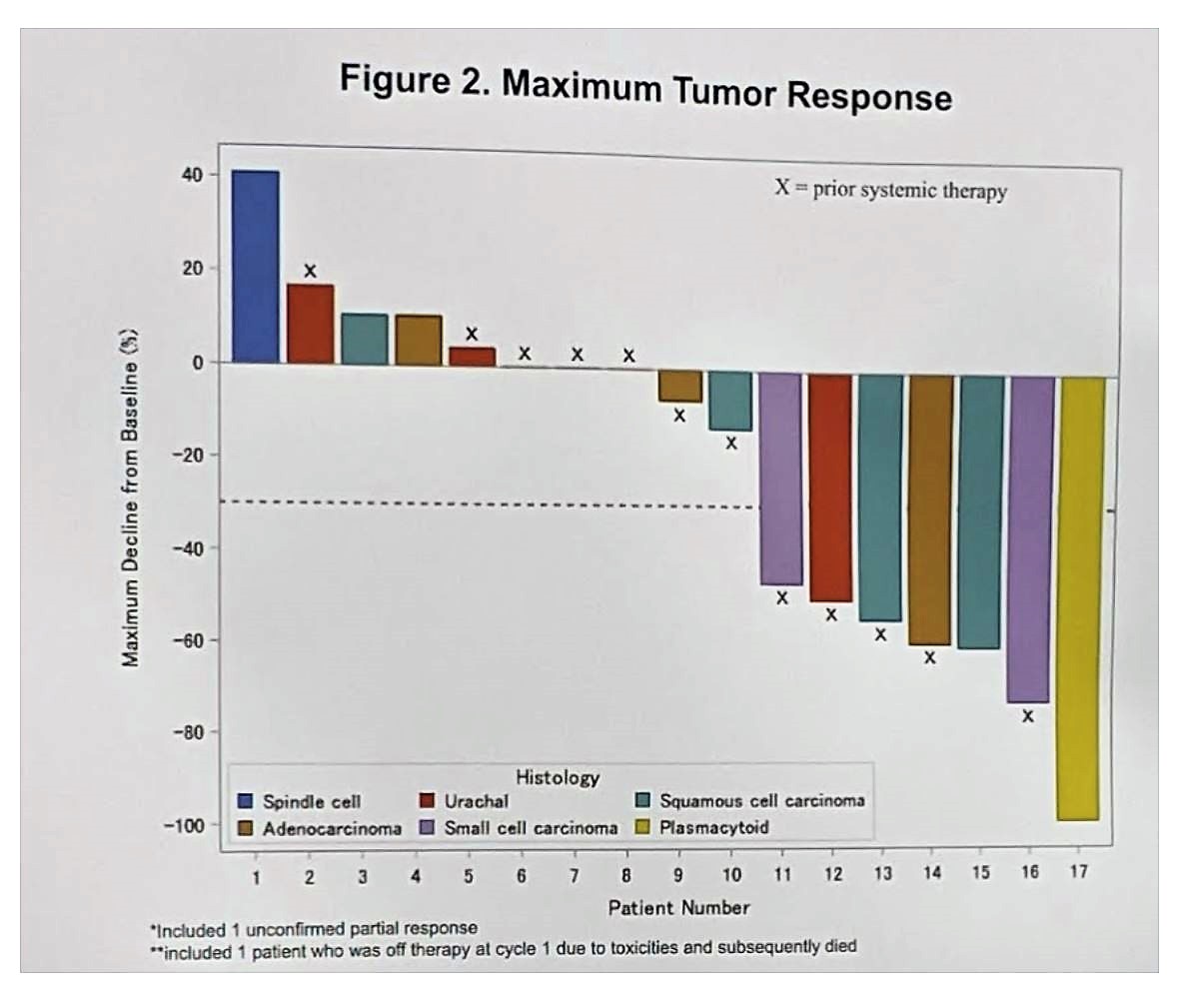
The fully accrued cohort included 19 BCVH patients enrolled at 4 institutions between 4/2018 and 1/2019; these included squamous cell (n = 6), small cell (n = 3), adenocarcinoma (n = 3), urachal (n = 5), plasmacytoid (n = 1), and spindle cell (n = 1) histologic subtypes. 13 (68%) patients had received prior systemic therapy, which entailed platinum-based chemotherapy in 92% of patients. Full demographics are seen below:

The median number of cycles of ipilimumab plus nivolumab received amongst the 19 patients was 4 (range 1-11). 9 of 19 patients remain on therapy, 10 were discontinued (8 for progression, 1 for toxicity, 1 for treatment-related death). Of the 19 patients, objective response rate (ORR) was 37% (7/19), complete response (CR) was seen in 1 patient (plasmacytoid histology). Disease control rate was 58% (11/19). ORR seemed to be highest in small cell and plasmacytoid patients, albeit in low numbers.

Maximal tumor response, by the patient, is seen below:
Kaplan Meier (KM) curve for progression-free survival (PFS) is seen below, and the median PFS is 3.8 months:

6-month overall survival (OS) KM curve is seen below:

At 6-month, OS is ~81%.
As for side effects, 5 patients developed treatment-related grade 3 toxicities, 2 developed grade 4 toxicity, and 1 patient died of treatment-related toxicity. Overall, this combination appeared to be well tolerated – consistent with prior studies assessing this combination.
As a trial that specifically focusing on rare GU cancers, nivolumab and ipilmumab resulted in promising objective responses in the cohort of patients with BCVH. The toxicity profile was manageable. As such, this combination may warrant further investigation in patients with BCVH, which has substantial unmet needs.
Clinical trial #: NCT03333616.
Presented by: Bradley Alexander McGregor, MD, Chair, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA


