The key inclusion criteria included:
- Age ≥18 years
- Histological evidence of metastatic or surgically unresectable locally advanced urothelial carcinoma (cT4b, or any N+ [N1–3] or any M-1 disease)
- Measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1
- Progression or recurrence after ≥1 platinum-based regimen for metastatic disease or surgically unresectable locally advanced urothelial carcinoma or within 12 months of perioperative (neoadjuvant or adjuvant) platinum treatment for muscle-invasive disease
- Eastern Cooperative Oncology Group (ECOG) performance status of ≤1
- Availability of an assessable tumor sample for biomarker analysis
- Serum creatinine ≤1.5 times the upper limit of normal or creatinine clearance ≥30 mL/min
The primary endpoint was objective response rate (ORR) (including DoR) by blinded independent review committee (BIRC) per RECIST v1.1 in all treated patients and by tumor programmed death ligand 1 (PD-L1) expression (1% and 5% cutoffs). The secondary endpoints included progression-free survival (PFS) per BIRC, overall survival (OS), and investigator assessed ORR. Other secondary outcomes included PFS per investigator assessment and safety.
The CheckMate 275 demonstrated the therapeutic advantage of nivolumab monotherapy in metastatic urothelial carcinoma patients who are refractory to 1st line platinum-based chemotherapy. Based on this study, nivolumab was approved for the treatment of patients with locally advanced or metastatic platinum-resistant urothelial carcinoma in the United States and Europe.3,4
In the CheckMate 275 trial, after a minimum follow-up of 21 months, objective response rate (ORR) was 20.4% and responses were durable. The median duration of response (DoR) was 17.7 months (95% CI, 11.5–22.0). Median overall survival (OS) was 8.6 months (95% CI, 6.1–11.3), with a manageable safety profile.5 Complete response (CR) was achieved in 17 (6.3%) patients. In the presented study, the authors report updated efficacy and safety results from CheckMate 275 with an extended minimum follow-up of 33.7 months.
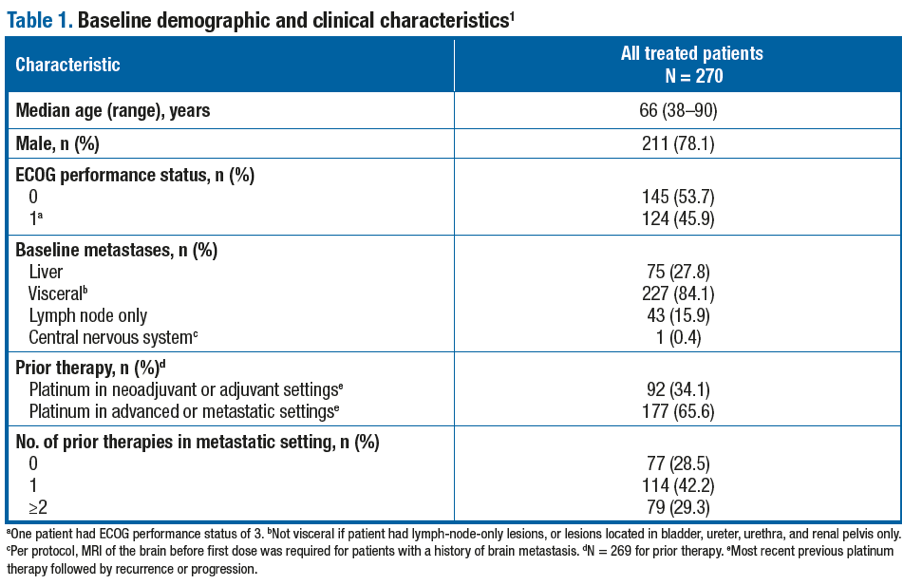
Baseline demographic and clinical characteristics are shown in Table 1. The ORR (95% CI) per BIRC was 20.7% (16.1–26.1), including 18 (7%) complete response (Table 2).
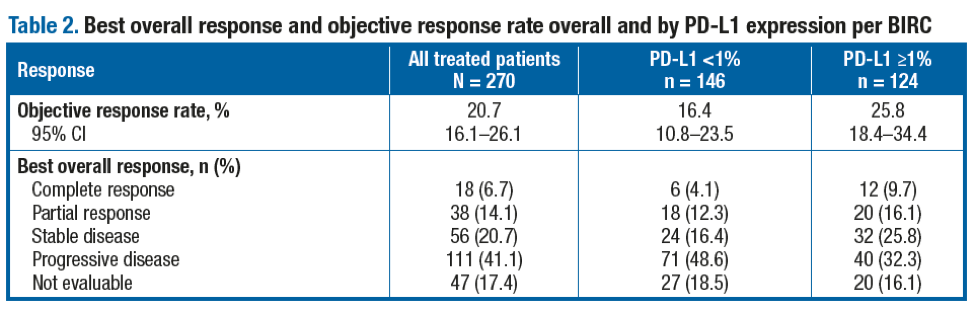
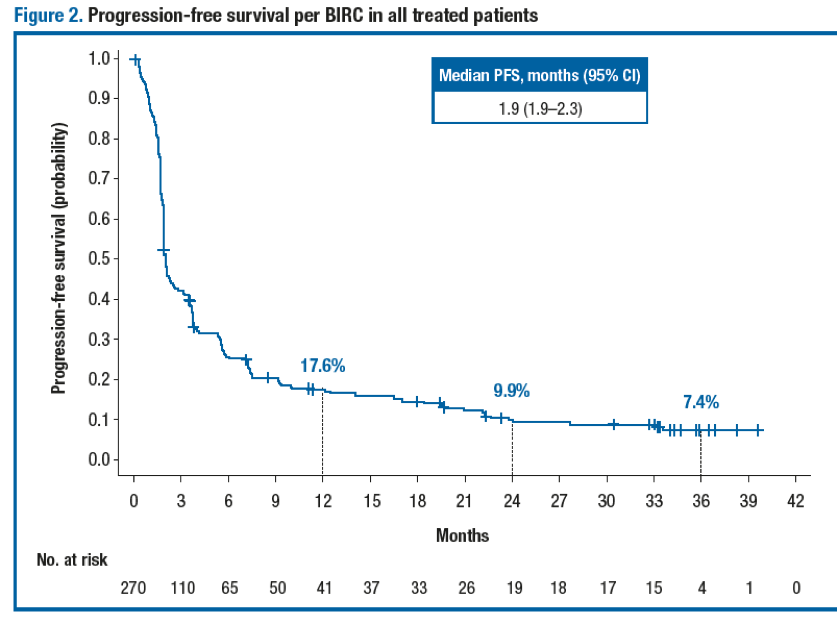
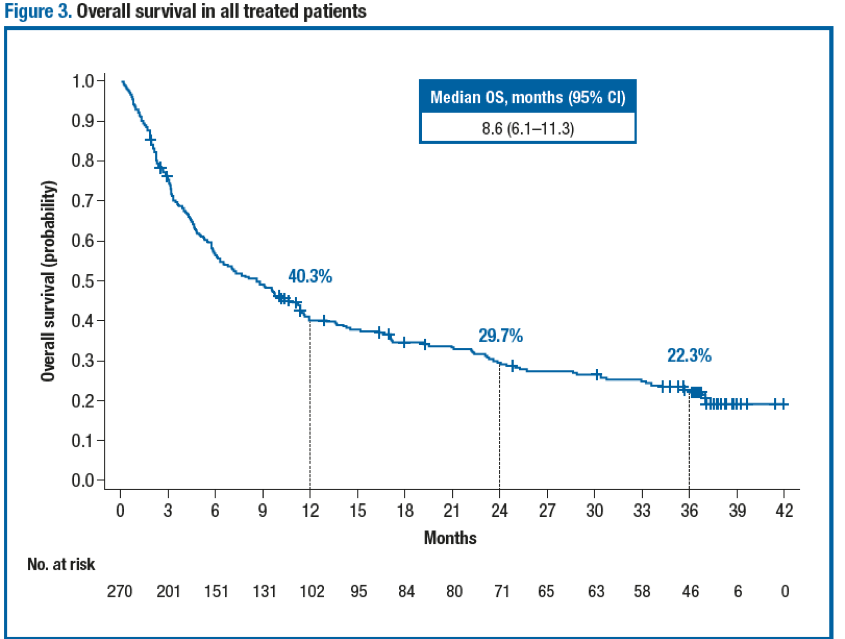
Antitumor activity was observed regardless of baseline tumor PD-L1 expression level at the 1% (Table 2) and 5% cutoffs. The ORR (95% CI) per investigator in all treated patients was 24.8% (19.8–30.4). The median time to response per BIRC in all treated patients was 2.0 months (range, 1.6–13.8). The median DoR (95% CI) per BIRC in all treated patients was 20.3 months (11.5–31.3). Importantly, the median PFS (95% CI) per BIRC in all treated patients was 1.9 months (1.9–2.3) (Figure 2), while the median PFS was 1.9 months (1.7–2.0) in patients with baseline PD-L1 expression <1% and 3.5 months (1.9–3.7) in patients with baseline PD-L1 expression ≥1%. The median OS (95% CI) in all treated patients was 8.6 months (6.1–11.3) (Figure 3), and the median OS was 6.0 months (4.4–8.1) in patients with baseline PD-L1 expression <1% and 11.9 months (9.1–19.1) in patients with baseline PD-L1 expression ≥1%.
When analyzing the safety details, any-grade treatment-related adverse-event occurred in 69.3% of patients, but grade 3–4 treatment-related AEs occurred in 24.8% of patients, and grade 5 treatment-related AEs occurred in 3 patients. The incidence of treatment-related adverse events generally decreased over time (Figure 5). The most common any-grade treatment-related select adverse events were skin (23.7%) and gastrointestinal (13.3%) events. Most treatment-related select adverse events (83.3%) resolved with a median time to resolution of patients with gastrointestinal adverse effects (3.1 weeks; 1.6–4.3), 35.7% of patients with hepatic adverse effects (Not reached), 57.1% of patients with pulmonary adverse effects (16.1 weeks; 4.0–41.4), 75.0% of patients with renal adverse effects (14.6 weeks; 1.6–42.1), and 58.7% of patients with skin adverse events (25.4 weeks; 13.1–NE).

In summary, CheckMate 275 shows that nivolumab continues to provide durable antitumor activity in patients with metastatic urothelial carcinoma, showing potential for additional complete response over time. PFS and OS continued to be maintained with extended follow-up. Importantly, the efficacy was observed regardless of PD-L1 expression. No new safety signals were identified compared with earlier reports (1,4). These data support further investigation of nivolumab in bladder cancer. There are several ongoing clinical trials investigating nivolumab, including:
- CheckMate 901 (in combination with ipilimumab for untreated metastatic urothelial carcinoma; NCT03036098)
- CheckMate 274 (as monotherapy for muscle-invasive bladder cancer in the adjuvant setting; NCT02632409)
- CheckMate 9UT (alone or in combination with BMS-986205 with or without bacillus Calmette–Guérin in non–muscle-invasive bladder cancer; NCT03519256)
- A phase 3 randomized study of neoadjuvant chemotherapy alone or with nivolumab ± BMS-986205 in muscle-invasive bladder cancer (NCT03661320)
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Suh J, Kim KH, Lee SH, et al. Prevalence and management status of urologic disease in geriatric hospitals in South Korea: A population-based analysis. Investig Clin Urol. 2017 Jul;58(4):281-288. doi: 10.4111/icu.2017.58.4.281. Epub 2017 Jun 27.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 Mar;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7. Epub 2017 Jan 26.
- OPDIVO (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2018.
- OPDIVO. Available at https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo. Accessed April 15, 2019.
- Sharma P, et al. Oral presentation at AACR 2018; April 17, 2018; Chicago, IL. Abstract CT178.


