BCG unresponsiveness to treatment includes:
- BCG refractory - Stage progression at 3 months after adequate BCG induction, or persistent high risk NMIBC at six months despite adequate BCG
- BCG relapsing – Recurrence of high risk NMIBC after the patient achieves a disease -free state within 12 months after adequate BCG therapy.
There is an urgent need for novel therapies to reduce the risk for recurrence and preserve the bladder. Because of the lack of a suitable comparator, single arm-design trials to test novel agents are acceptable in the BCG-unresponsive population.5
Programmed death 1 (PD-1) pathway activation has been implicated in BCG resistance.9 Pembrolizumab is a highly selective humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1, and PD-L2. It has shown significant and durable antitumor activity in metastatic urothelial carcinoma.10,11 There is evidence showing that the overall survival is better with pembrolizumab than chemotherapy in platinum-refractory disease.11 Due to the limited knowledge on the effect of anti-PD1 monotherapy for NMIBC, this study was conducted.
The objective of this study was to evaluate the antitumor activity of pembrolizumab monotherapy among patients with CIS at baseline. The authors also aimed to evaluate the safety and tolerability of pembrolizumab monotherapy among patients with high-risk NMIBC (Figure 1).
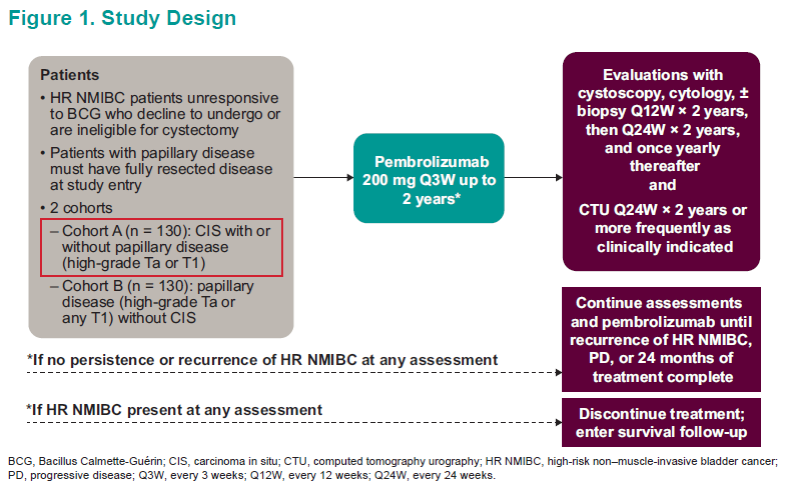
The primary endpoint was the rate of complete response (CR) of high-risk NMIBC in patients with CIS at baseline. Complete response was defined as the absence of high-risk MIBC or absence of progressive disease by central review. The secondary endpoints included complete response of any disease, duration of response (DOR), and safety/tolerability
A total of 102 patients were included and analyzed. The patient disposition and baseline characteristics are shown in Figure 2 and Table 1.
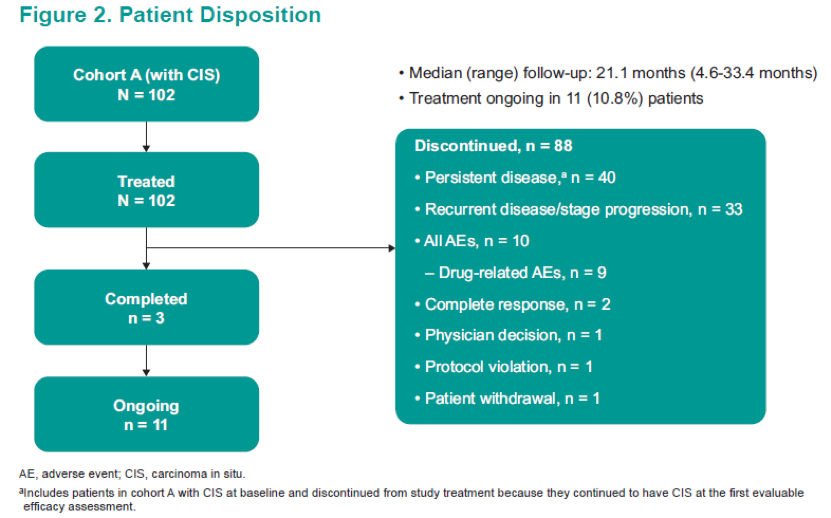


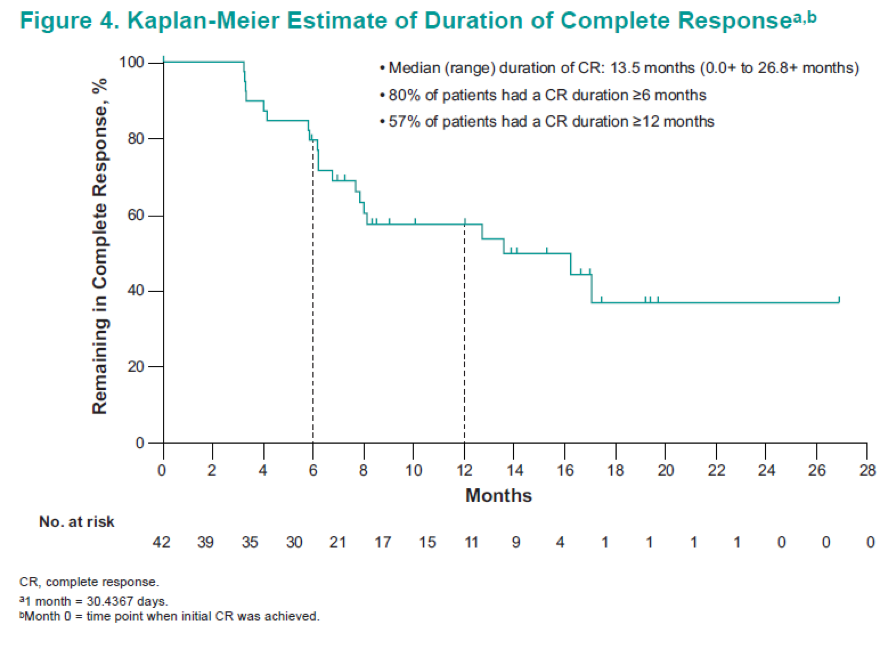
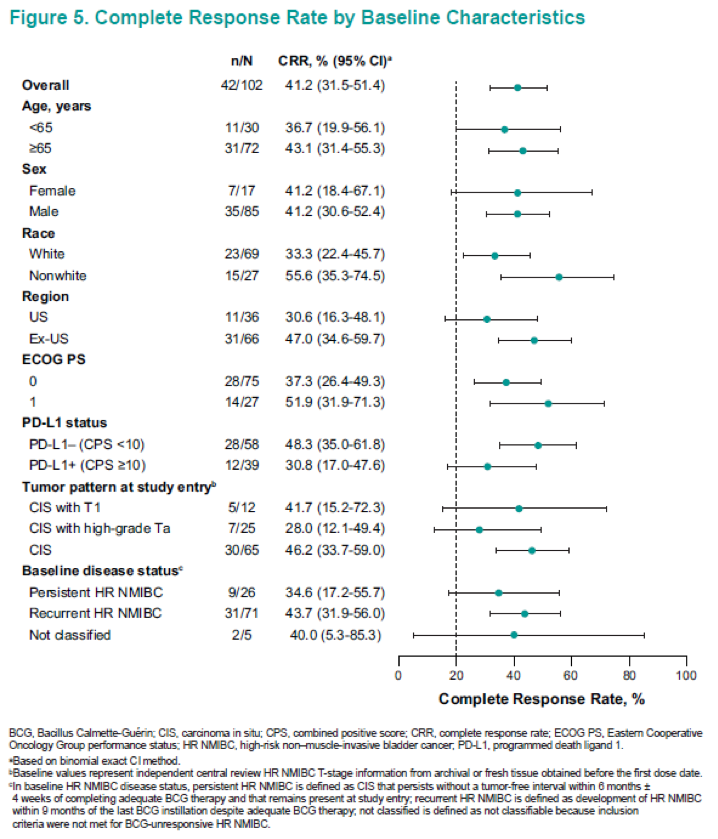
In summary, in this updated analysis of 102 patients from this ongoing study, pembrolizumab monotherapy continued to demonstrate encouraging antitumor activity in patients with BCG-unresponsive CIS (with or without papillary disease) who refused to undergo or were ineligible for radical cystectomy. The complete response rate was 41.2% with a median duration of 13.5 months. None of the patients progressed to muscle-invasive disease or metastasis while on therapy. The pembrolizumab profile was consistent with profiles shown in previous studies of this drug.10,11
There is currently a phase 3 study enrolling patients to evaluate the efficacy and safety of pembrolizumab + BCG in high risk NMIBC that is persistent/recurrent after BCG induction (KEYNOTE-676, NCT03711032). We eagerly await the results of this trial, and its implications for high risk NMIBC patients.
Presented by: Ronald De Wit, MD, PhD, Erasmus University Medical Center, Rotterdam, Netherlands
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Cumberbatch MGK et al. Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol 2018
- NCCN guidelines: bladder cancer version 3. 2019
- Hemdan T et al. J Urol 2014
- Herr HW et al. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol 2015
- Anastasiadis A et al. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol 2012
- Kamat et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. JCO 2016
- US department of health and human services: BCG-unresponsive NMIBC: developing drugs and biologics for treatment – guidance for industry.
- Babjuk M et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017
- Fukumoto K et al. Clinical Role of Programmed Cell Death-1 Expression in Patients with Non-muscle-invasive Bladder Cancer Recurring After Initial Bacillus Calmette-Guérin Therapy.Ann Surg Oncol 2018
- Balar AV et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017
- Bellmunt J et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. NEJM 2017


