In preclinical models, metformin, a commonly used oral diabetes medication, has demonstrated anti-neoplastic effects in many solid tumors including colon cancer, pancreatic cancer, and breast cancer.3 Its mechanism of action is thought to be related to activation of the AMP-activated protein kinase (AMPK) pathway, inhibiting the expression of genes involved in cell cycle regulation and mitosis. The relationship between metformin and prostate cancer is controversial. Preclinic studies in prostate cancer cell lines have suggested that metformin is able to reduce cyclin D1 activity, activate the AMPK pathway, and inhibit mTOR signaling.



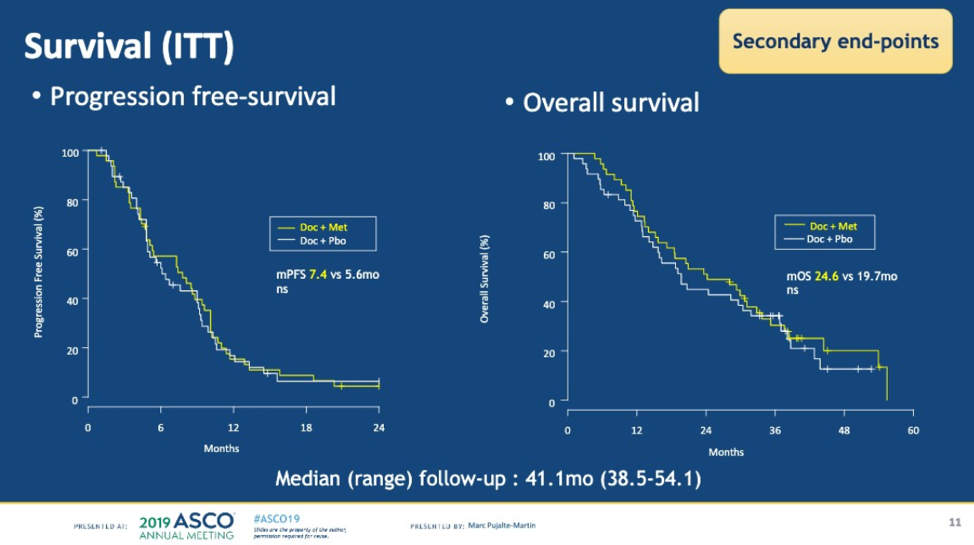
In terms of the primary endpoint, there was no difference in PSA50 (72% in both arms). In terms of the secondary objectives, there was also no difference in objective response rates (28% in both arms), clinical median progression-free survival (mPFS) (7.3 months vs 5.8 months p = 0.848), and no difference in median overall survival (24.2 months vs 19.7 months, p = 0.53). Patients receiving metformin had a higher incidence of diarrhea which is a known side effect of metformin.


Presented by: Marc Pujalte Martin, MD, Centre Antoine Lacassagne, Nice, France
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Kyriakopoulos CE, Chen Y-H, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. Journal of Clinical Oncology 2018;36:1080.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. New England Journal of Medicine 2004;351:1502-12.
- He K, Hu H, Ye S, Wang H, Cui R, Yi L. The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Scientific reports 2019;9:2218.


