Apalutamide and enzalutamide have demonstrated improvements in metastasis-free survival (MFS) in nmCRPC, but are associated with increased rates of fatigue, falls, fractures, mental impairment, and other adverse events compared with placebo.2,3 The nmCRPC patient population is largely asymptomatic and would benefit from treatments that delay disease progression with minimal all-grade adverse events.1
Generally speaking, nmCRPC patients are concerned with four important factors:
- Quality of life
- Symptoms
- Survival
- PSA and related anxiety
Figure 1 – ARAMIS trial design:

Quality of life was assessed (preplanned) with four validated questionnaires:
- BPI-SF
- FACT-P
- EQ-5D-3L
- EORTC-QLQ-PR25
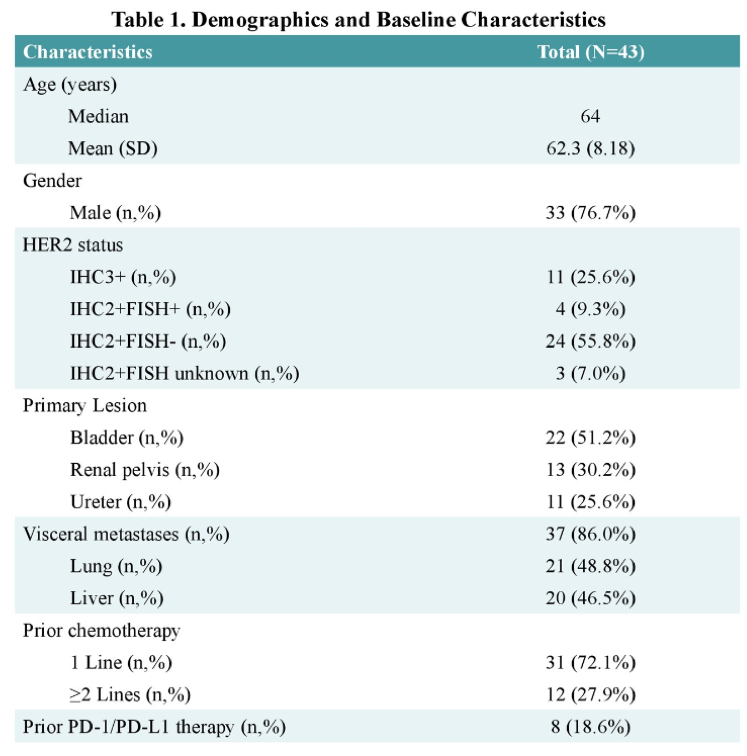
The baseline characteristics were the same in both trial arms (Table 1). The results were consistent in almost all endpoint and quite impressive. MFS and OS were significantly better in the Darolutamide arm (40.4 vs. 18.4 months for MFS) (Figure 2). Similarly, the time to PSA progression was significantly better with Darolutamide, compared to placebo, with an astounding hazard ratio of 0.13 (95% CI 0.11-0.16) (Figure 3).
Table 1 – Baseline characteristics of patients in the trial arms:
When analyzing the incidence of treatment-emergent adverse events, a similar percentage of patients had discontinued treatment in both arms (8.9% vs. 8.7%), and more urinary adverse effects had occurred in the placebo arm (4.9% vs. 3.5% urinary infection, and 6.3% vs. 2.5% urinary retention).
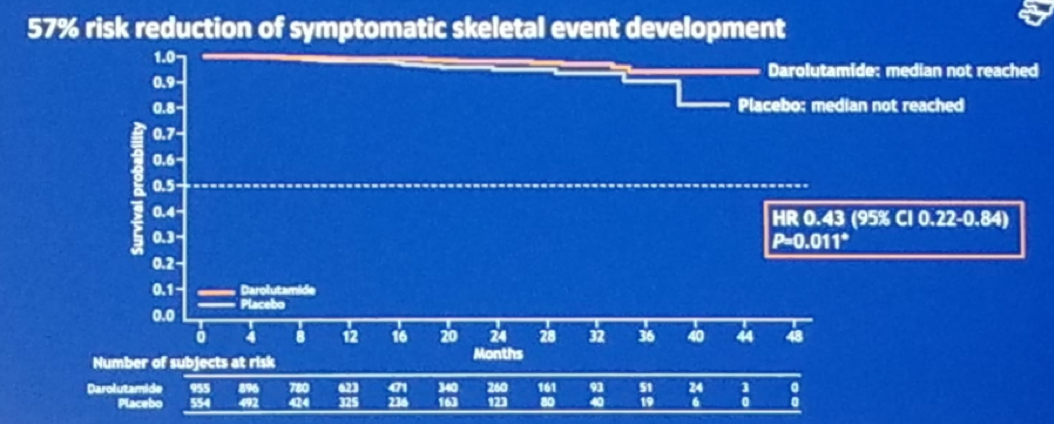
Another secondary endpoint was time to pain progression, and the results again showed a benefit in the Darolutamide arm (Figure 4). Time to first symptomatic skeletal event was not completely mature, as the median was not yet reached, but the Darolutamide arm showed an advantage in this as well. (Figure 5).
Figure 2 – Metastasis-free survival and overall survival:
Figure 3 – Time to PSA progression:

Figure 4 – Time to pain progression:

Figure 5 — Time to symptomatic skeletal-related event:
When assessing the quality of life using the FACT-P method, it was maintained throughout the study, but again showed a benefit in the Darolutamide arm with an HR of 0.8 (95% CI 0.70-0.91, p=0.0005). Examining the time to deterioration of EORTC-QLQ-PR25 subscales, Darolutamide demonstrated clinically significant delays in time to deterioration compared with placebo for urinary and bowel symptoms. The time to deterioration of the other subscales was not statistically different between groups.
Dr. Fizazi concluded his talk, stating that Darolutamide significantly improves MFS in nmCRPC patients. This drug has a favorable safety profile and maintains a good quality of life in these patients. Summing all these results, Darolutamide seems to be an attractive option for nmCRPC patients.
Presented by: Karim Fizazi, MD, Ph.D, Medical Oncologist, Head of the Department of Cancer Medicine, Institut Gustave Roussy, Villejuif, France, Professor of Oncology, University of Paris, Paris, France
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Mateo J. et al. "Managing Nonmetastatic Castration-resistant Prostate Cancer." European Urology. 2019. DOI: https://doi.org/10.1016/j.eururo.2018.07.035
- Smith MR, et al. "Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer." New England Journal of Medicine. 2018. DOI: 10.1056/NEJMoa1715546
- Hussain M. et al. "Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer." New England Journal of Medicine. 2018. DOI: 10.1056/NEJMoa1800536.
- Fizazi K et al. "Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer." New England Journal of Medicine. 2019. DOI: 10.1056/NEJMoa1815671.


