Figure 1 – PSMA enzyme:

Since 2006 there has been a rise in the number of studies published on the usage of PSMA-PET, with a significant increase since 2016. The current updated European Association of Urology guidelines recommend using PSMA PET scan in patients with persistent PSA after radical prostatectomy. However, the strength rating of this recommendation is still “weak” due to lack of knowledge regarding the effect of using this imaging modality on outcomes.
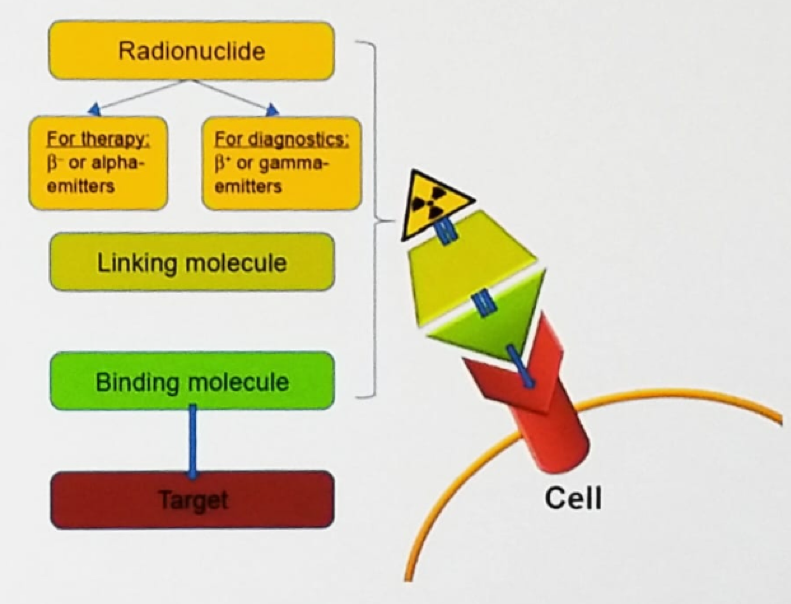
Theranostics is a novel and growing field of medicine, combining specific targeted therapy based on specific diagnostic tests, with a key focus on patient-centered care. Theranostics provides a transition from conventional medicine to a contemporary personalized and precision medicine approach. The theranostic approach in nuclear medicine combines diagnostic imaging and therapy using the same molecule or very similar molecules (Figure 2), which are either radiolabeled differently or given in different dosages. For example, iodine-131 and lutetium-177 are gamma and beta emitters. Thus, these agents can be used for both imaging and therapy. In this approach, only what you can see will be treated so it is important to identify all disease sites.
Figure 2 – Theranostic approach in nuclear medicine:
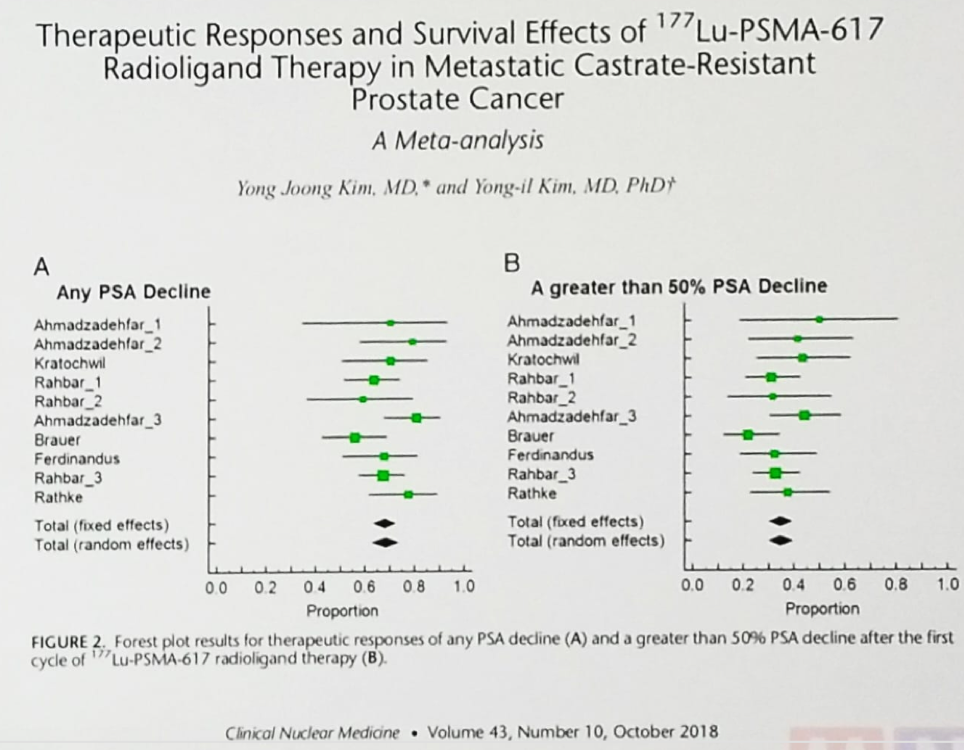
In 2011 the first patient was treated with PSMA radioligand therapy (131I), and in 2014, the first patient was treated with 177Lu-PSMA-617. Since 2015 there have been plenty of single-center small cohort studies examining the treatment with 177Lu-PSMA-617. These have shown promising response rates, a favorable safety profile and some preliminary data on clinical outcomes. In a meta-analysis of 177Lu-PSMA-617 in patients with metastatic castrate-resistant prostate cancer (mCRPC) any PSA response was seen in close to 70% of patients, and a PSA response of more than 50% was seen in almost 40% of patients (Figure 3).1
Figure 3 – Meta-analysis of 177Lu-PSMA-617 in patients with metastatic castrate-resistant prostate cancer:
In a phase two, single-arm, single-center trial assessing the role of 177Lu-PSMA-617 in patients with mCRPC, published in Lancet Oncology2 in 2017, 50% of the patients had more than a 50% PSA response, and 27% of the patients had more than an 80% PSA response, as seen in figure 4. These results are quite encouraging, but there is still more room for improvement.
Figure 4 – Phase two, single-arm, single-center trial assessing the role of 177Lu-PSMA-617 in patients with mCRPC :

Dr. Fanti concluded his presentation stating that in properly preselected patients, targeted nuclear therapies have proven to be effective with a favorable safety profile. The combination of targeted cancer imaging and therapy has a bright future and will most likely contribute to personalized medicine and play an increasingly important role.
Presented by: Stefano Fanti, MD, Director, Nuclear Medicine Division and PET Unit, The Policlinico S. Orsola, Director, Specialty School of Nuclear Medicine, University of Bologna, Bologna, Italy
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Kim YJ et al. "Radiation exposure after 177Lu-DOTATATE and 177Lu-PSMA-617 therapy." Clinical Nuclear Medicine. 2017. doi: 10.1007/s12149-018-1264-x
- Hoffman MS. Et al. "[177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study." Lancet Oncology. 2018. doi: 10.1016/S1470-2045(18)30198-0.


