- Pembrolizumab + Olaparib
- Pembrolizumab + Docetaxel + Prednisone
- Pembrolizumab + Enzalutamide
- Pembrolizumab + Abiraterone.
This abstract describes the outcomes of 69 patients who were treated with the combination of pembrolizumab and enzalutamide. All patients had progressed on abiraterone for mCRPC. Patients received pembrolizumab 200 mg daily in combination with enzalutamide 160 mg daily. The median age of all patients was 69 and all patients had an ECOG of 0 or 1. The majority of patients were deemed PD-L1 negative (CPS<1) and 26% of patients had visceral disease.
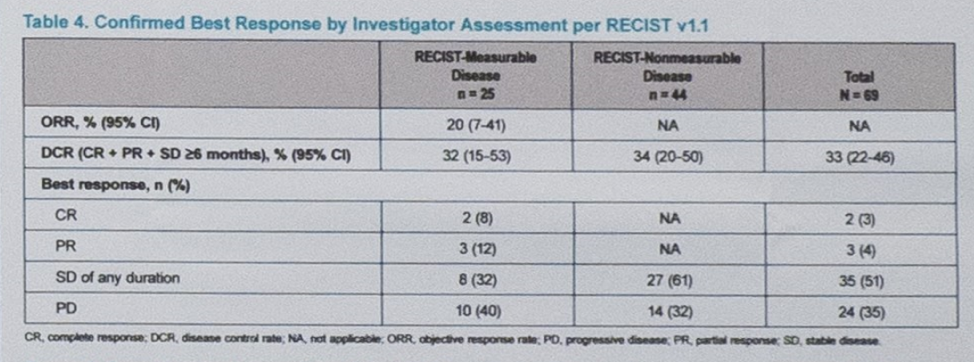
For patients with measurable disease, the objective response rate (ORR) was 40% (10/25), and the ORR for the total evaluable population was 27% (18/67). The median radiographic progression-free survival (rPFS) was 6 months and the median overall survival has not yet been reached. Of the patients with objective responses, the median duration was 8.3 months with 75% of patients having a response greater than 6 months.

Presented by: Peter C. Fong, MBBS, FRACP, Auckland Hospital, New Zealand
Written by: Jason Zhu, MD, Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- De Bono JS, Goh JC, Ojamaa K, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2018.
- Sandhu GS, Parikh RA, Appleman LJ, Friedland D. Enzalutamide after abiraterone in patients with metastatic castrate-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2014.


