The median overall survival for the entire group was 4.5 years from the time of mCRPC designation from the original abstract. Genes of interest included PTEN, TP53, RB1, BRCA2, ATM, AR amplification, and AR mutation. The NHT group as a whole had a median time to treatment failure of 12.2 months and the median time to treatment failure (TTTF) of docetaxel was 5.1 months.
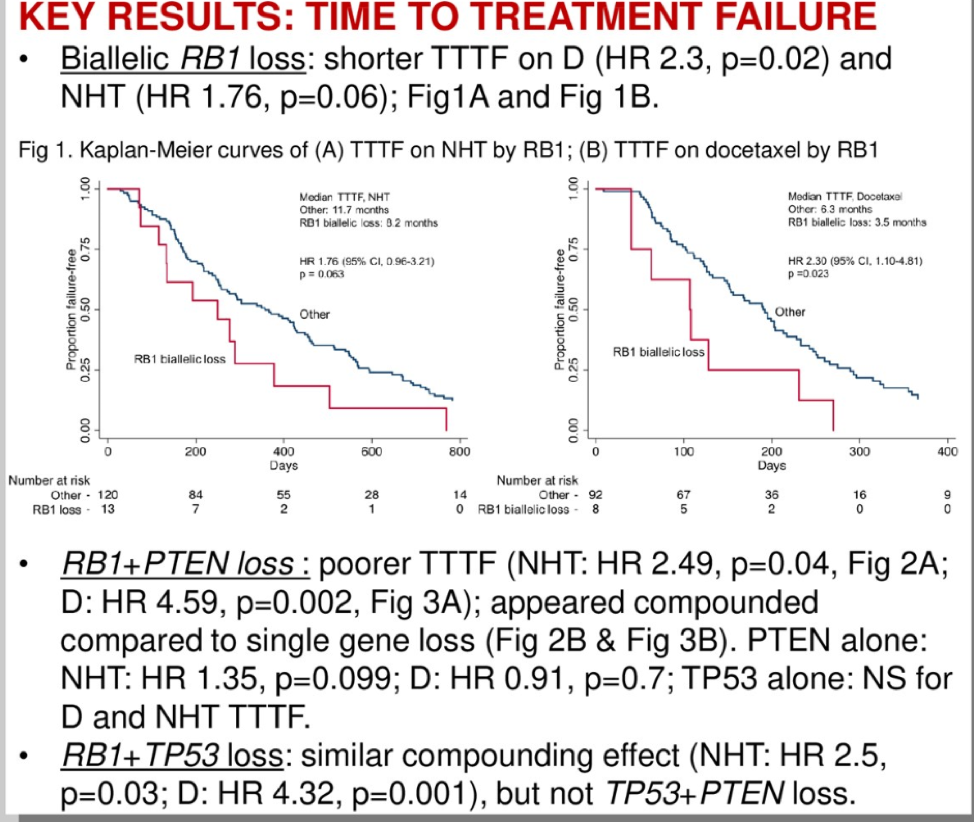
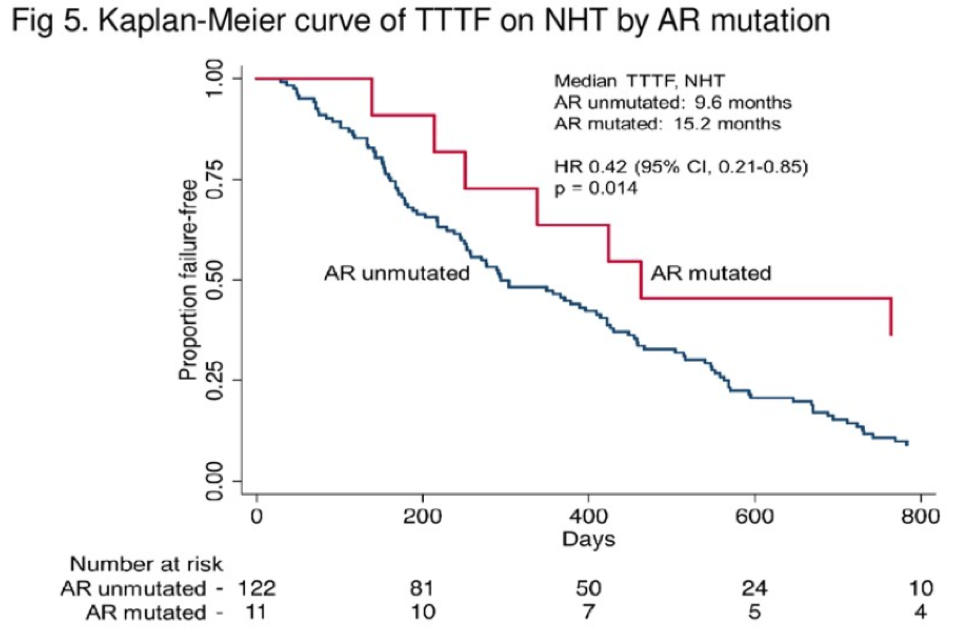
This exploratory analysis found biallelic RB1 loss is present in 12% of tumors and these patients have a shorter TTTF with either hormonal therapy or chemotherapy. If patients also had an additional PTEN or TP53 loss, this would worsen prognosis and survival. It is unclear what the sequencing of therapies was for these patients, as many patients received both NHT and docetaxel and this sequencing may impact the TTTF. Regardless, this analysis is thought-provoking and future studies should prospectively incorporate genomic biomarker analysis so we can better identify which therapies are most beneficial for our patients at the right time.
Presented by: Anis Hamid, MBBS, Medical Oncologist, Melbourne Australia, GU Oncology Research Fellow, Dana-Farber Cancer Institute Boston, Massachusetts
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Sircar K, Yoshimoto M, Monzon FA, et al. PTEN genomic deletion is associated with p‐Akt and AR signaling in poorer outcome, hormone refractory prostate cancer. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 2009;218:505-13.


