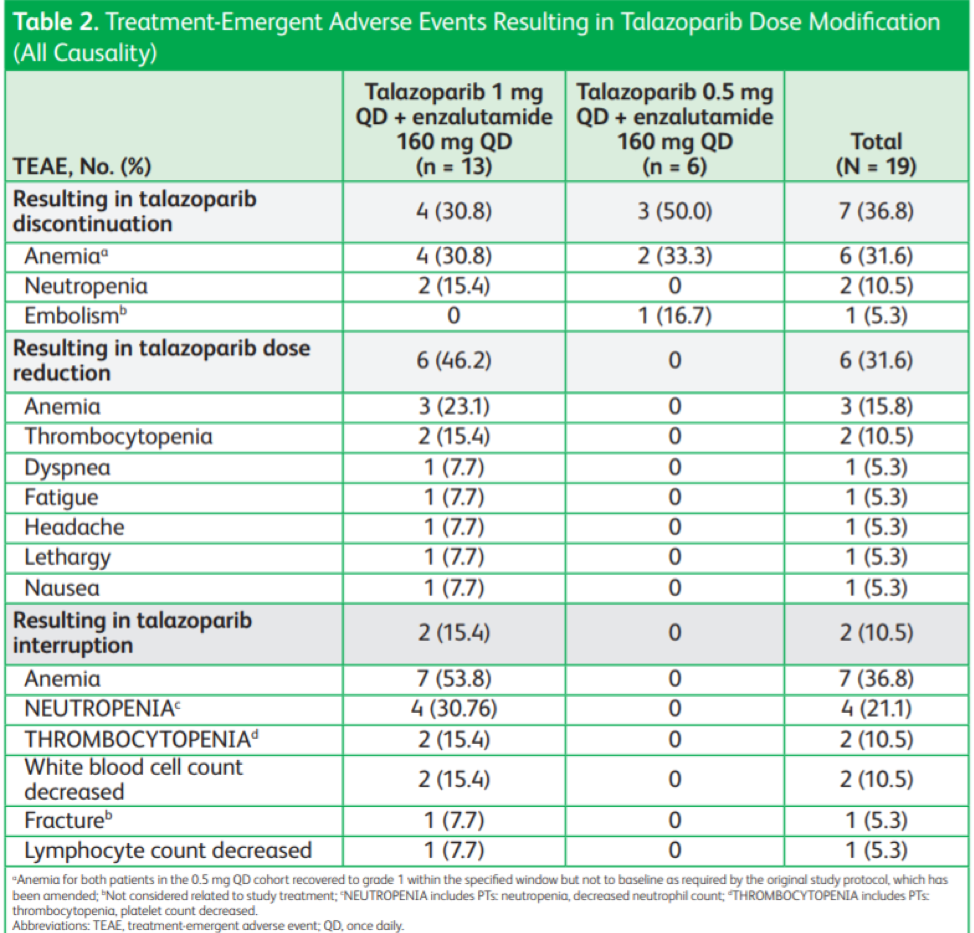
This abstract provides data on 19 patients with mCRPC who were enrolled in two dose cohorts – 13 patients received 1 mg and 6 patients received 0.5 mg talazoparib daily. At the time of data cutoff, the median treatment duration was 11 weeks for the 1 mg cohort and 11 weeks for the 0.5 mg cohort. Treatment-emergent adverse events occurred in all patients, the most common being anemia, which occurred in 76.9% of patients in the 1 mg cohort and 33.3% in the 0.5 mg cohort. Anemia led to dose reductions in 47.2% of the patients in the 1 mg cohort.
Overall, 18/19 (95%) of patients had a PSA50 and PK data showed that enzalutamide increased talazoparib exposure.

Talazoparib in combination with enzalutamide appears to be an effective therapy in mCRPC, with a 90% PSA50 rate in this small cohort of patients. Treatment adverse events (AEs) included anemia and neutropenia, both of which are expected with PARP inhibition. The 0.5 mg dose of talazoparib resulted in significantly fewer discontinuations than the 1 mg dose and will be the dose used in the randomized study. A large randomized study with over 800 patients is underway to determine if this combination is more effective than enzalutamide alone. Of note, all of these patients were treatment naïve and it is unknown if PARP inhibitors would improve the objective response rate in combination with enzalutamide if they had previously progressed on abiraterone or docetaxel. This would be interesting to evaluate for future studies.
Presented by: Neeraj Agarwal, MD, Associate Professor, Division of Oncology
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New England Journal of Medicine 2015;373:1697-708.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Oncology 2018.


