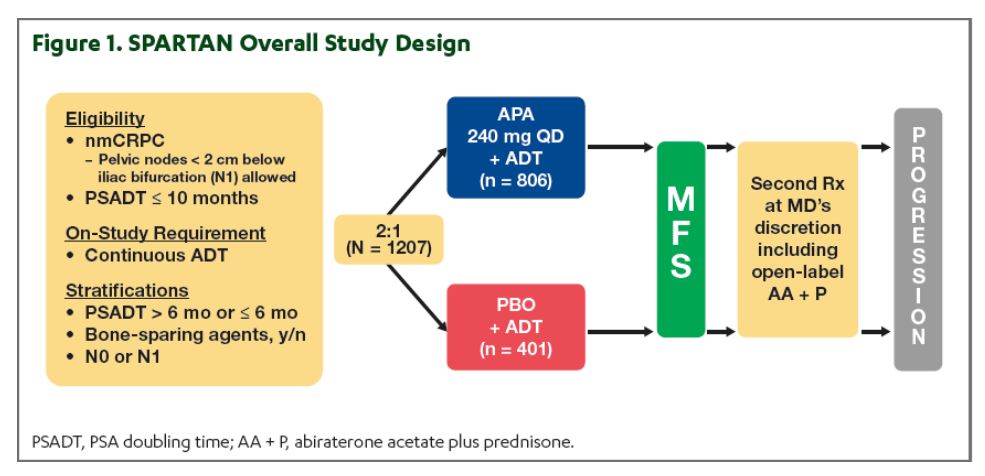
The SPARTAN trial (Clinical trial information: NCT01946204), is a randomized phase 3 placebo-controlled double-blind trial comparing apalutamide with long term androgen deprivation therapy (ADT) to placebo and long-term ADT in nmCRPC patients (Figure 1). This trial demonstrated that compared to placebo with ongoing ADT, apalutamide with ongoing ADT significantly prolonged metastasis-free survival (MFS), time to symptomatic progression, and secondary progression-free survival 5,6 with no decline in health-related quality of life.7 In the presented study, the authors assessed the impact of apalutamide on these endpoints specifically in patients with or without baseline comorbidities.
Figure 1 – SPARTAN trial design:
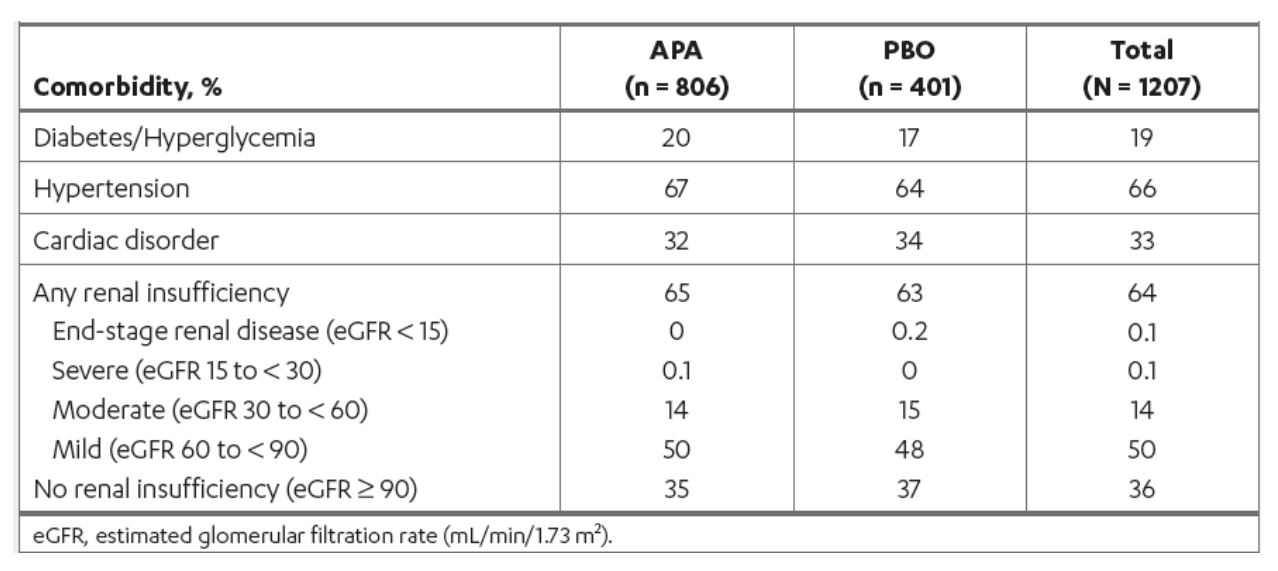
Patients in the SPARTAN study were stratified by the presence of baseline diabetes or hyperglycemia, cardiovascular disease, hypertension, and renal insufficiency, or by the number of comorbidities at baseline. Cox proportional hazards models were used to calculate hazard ratios for all study outcomes with and without underlying comorbidities. The incidences of any treatment-emergent adverse events were summarized by the presence of comorbidities by treatment group.
Of the total 1,207 SPARTAN patients, 1062 (88%) had more than one baseline comorbidity, including 703/806 (87%) patients in the apalutamide arm, and 359/401 (90%) patients in the placebo arm (Table 1). In general, patients with any underlying comorbidity were older and had greater incidences of Eastern Cooperative Oncology Group performance status (ECOG PS) = 1 (Table 2).
Table 1 – Percentage of patients with comorbidities in SPARTAN:

Table 2 – Baseline characteristics of SPARTAN patients:

The incidences of the specific comorbidities in both treatment groups are shown in Table 3.
Table 3 – Incidences of comorbidities in SPARTAN patients:
MFS was statistically improved in the apalutamide-treated patients compared to the placebo-treated patients in all comorbidity subgroups, and regardless of the number of comorbidities (Figure 2).
Figure 2- Metastasis-free survival in SPARTAN patients stratified by the presence of comorbidities:

Additionally, apalutamide-treated patients demonstrated a clinical benefit in the time to symptomatic progression compared to those treated with placebo, in all comorbidity subgroups (Figure 3). The treatment benefit of apalutamide on secondary progression-free survival was also statistically better in all comorbidity subgroups, except in patients who had diabetes/hyperglycemia (Figure 4). Lastly, Table 4 shows that a significant treatment benefit was maintained in all trial outcomes (MFS, symptomatic progression, and secondary progression-free survival) in the apalutamide treated patients, irrespective of how many comorbidities they had. The adverse events among apalutamide-treated patients were not affected by the presence of any of the evaluated comorbidities.
Figure 3- Time to symptomatic progression in SPARTAN patients by presence of comorbidities:
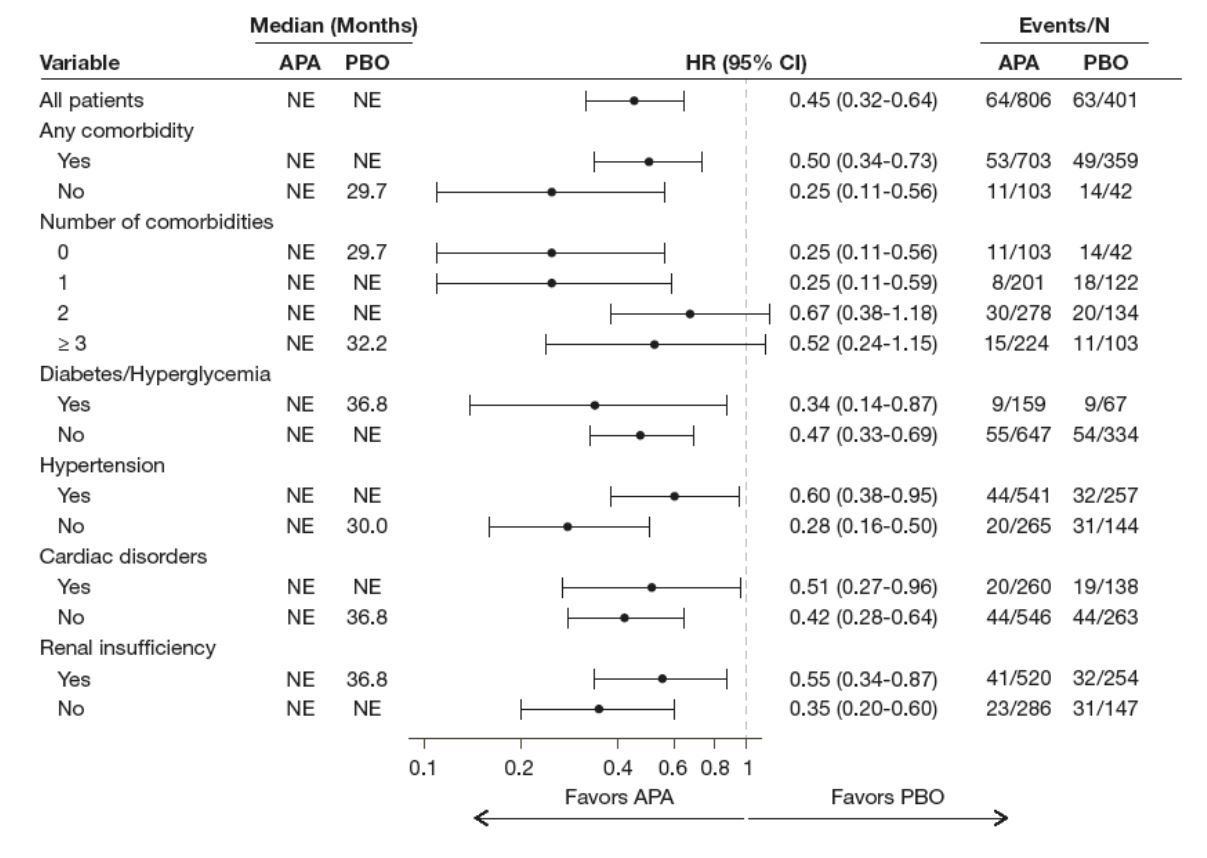
Figure 4 – Secondary progression-free survival in SPARTAN patients stratified by the presence of comorbidities:
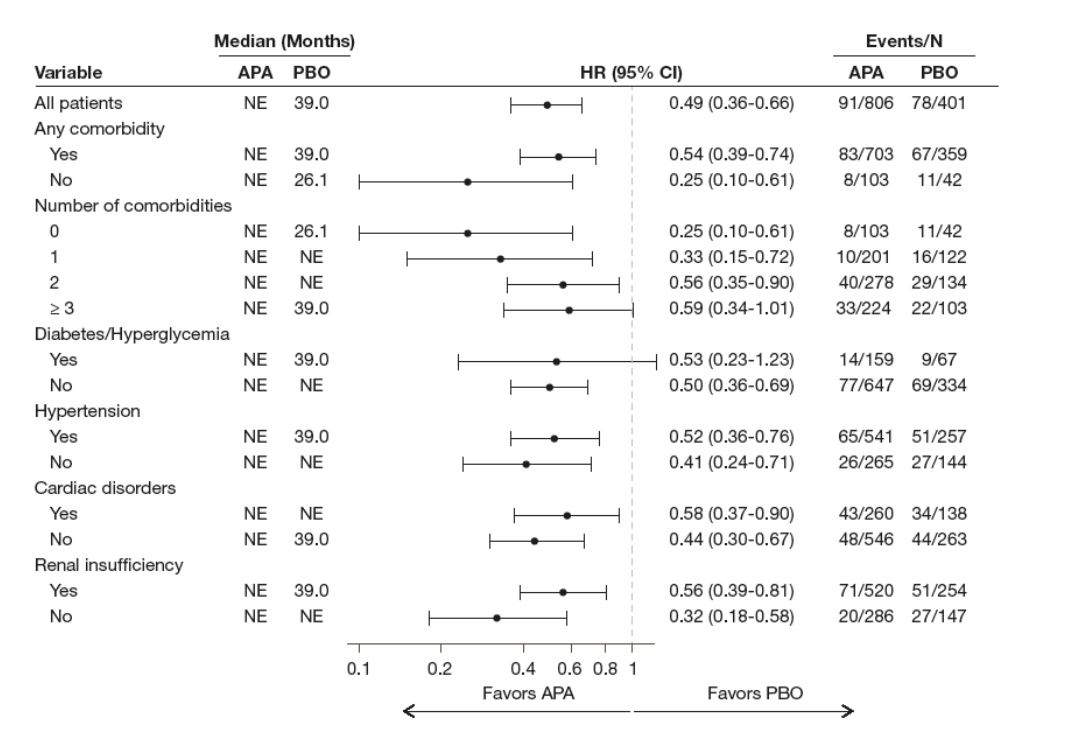
Table 4 – Apalutamide treatment benefit stratified by the presence of comorbidities:

In conclusion, in this presentation, the authors concluded that the benefit of apalutamide + ongoing ADT in patients with nmCRPC was maintained, regardless of the number of comorbidities. Furthermore, the safety profile of apalutamide was not affected by any comorbidity presence. This adds important information regarding treatment with apalutamide, making it a very attractive option for treating these patients.
Presented by: Eric Jay Small, MD, FASCO, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, California
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Smith MR, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. Journal of Clinical Oncology 2005 23:13, 2918-2925
- Smith MR, et al. Disease and Host Characteristics as Predictors of Time to First Bone Metastasis and Death in Men with Progressive Castration-Resistant Nonmetastatic Prostate Cancer. Cancer. 2011;117:2077-2085.
- Smith MR, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39-46.
- Lin JH, et al. Association of prostate specific-antigen doubling time (PSADT) with metastasis-free survival (MFS) and overall survival (OS) in non-metastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2017;35(15 suppl). Abstract e16525.
- Smith MR, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018;378:1408-1418.
- Small EJ, et al. Poster presented at the 2018 Genitourinary Cancers Symposium, February 8-10, 2018, San Francisco, CA. Abstract 161.
- Saad F, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1404-1416.


