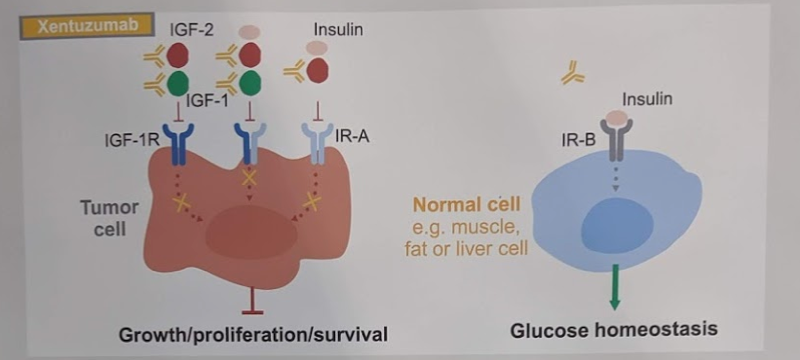
Enzalutamide (Enza), as an androgen-receptor axis targeted therapy, has known efficacy in the treatment of metastatic castration-resistant prostate cancer (mCRPC). Approved along with doxetaxel and abiraterone, it is often sequenced with these agents for the management of mCRPC patients.
In this multi-center randomized phase II trial, the authors evaluated anti-tumor activity of Xe plus Enza in mCRPC who have progressed on both docetaxel and abiraterone. Men with histologically/cytologically confirmed mCRPC and progression after docetaxel and abiraterone were randomized to receive Xe 1000mg IV QW + Enza 160mg/day oral or Enza alone (28-day cycles until progression or intolerable adverse events [AEs]). The study design is seen below:
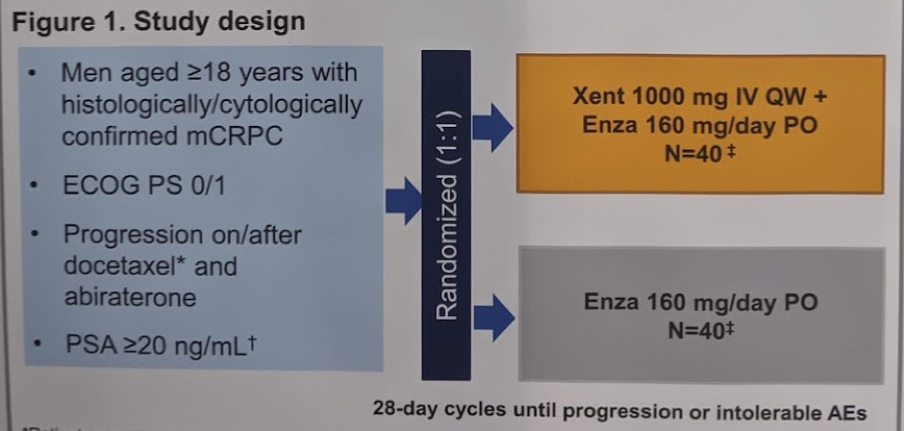
The primary endpoint was defined as progression-free survival by investigator assessment (PFS-IA), while secondary endpoints included PFS by central review (PFS-CR), overall survival (OS), AEs, and CTC response.
Overall, 43 patients were randomized per arm for a total of 86 patients. The table below summarizes the demographics:
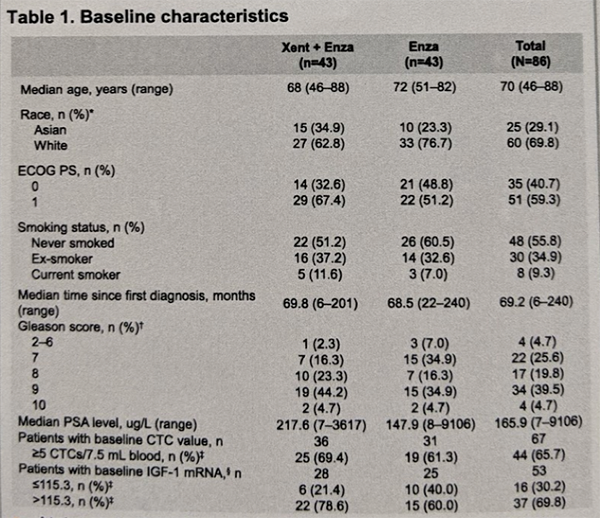
At baseline (BL) the two arms were generally well balanced, although 33% v 47% were ECOG PS O, and 72% v 56% had a Gleason total score ≥8.
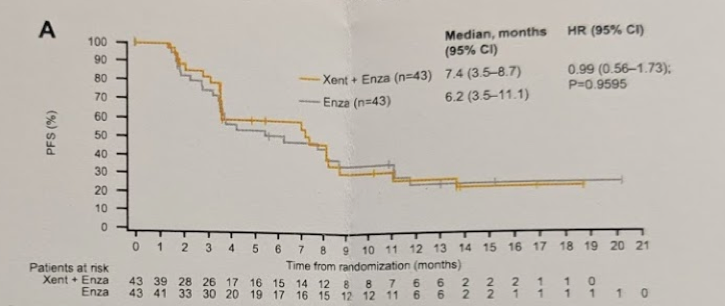
By data cut-off (23 October 2017), 39/43 Xe+Enza and 38/43 patients Enza had discontinued therapy, most due to disease progression. The median PFS-IA was 7.4 m for Xe+En (95% CI: 3.5–8.7) and 6.2 m for En (3.5–11.1) [HR = 0.99 (0.56–1.73); p = 0.96] – there was no significant difference between the two arms. The results remained similar after adjusting for BL ECOG PS and Gleason score.
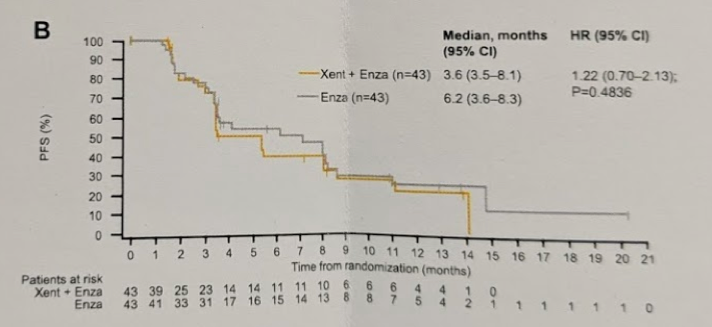
The median PFS-CR was 3.6 m for Xe+Enza (3.5–8.1) and 6.2 m for Enza (3.6–8.3) (HR = 1.22 [0.70–2.13]; p = 0.48) – again no significant difference.
Looking at PSA response, for the two arms, prostate-specific antigen (PSA) response rates were 21% and 19%; maximum decline in PSA: -20 v -9 μg/L. This is summarized below:

PSA change at week 12 was 19% v 18%, maximum decline in circulating tumor cells (CTC) was -52% v -35%, and CTC response was 16% v 11%. This is summarized below:
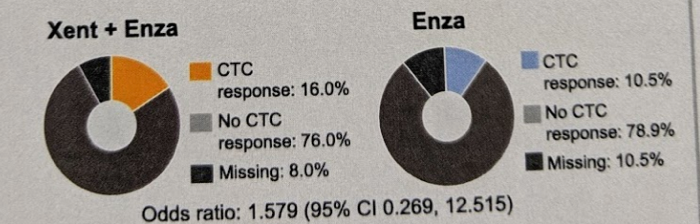
All of these were also not significantly different.
Time to PSA progression is seen in the KM curve below:
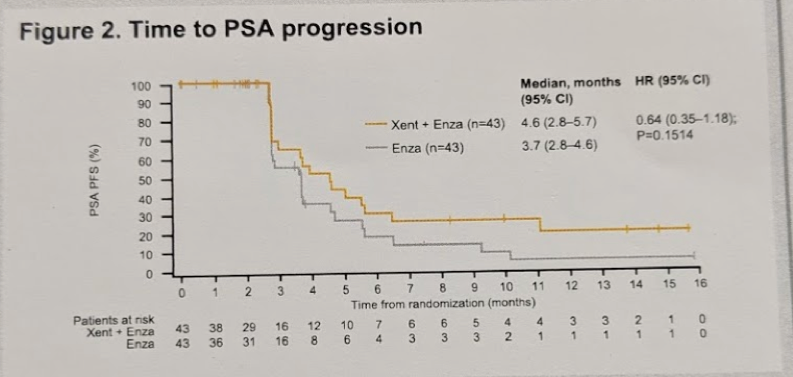
There was no significant difference between the two arms.
The most frequently reported AEs were: fatigue 67% v 49%; decreased appetite 56% v 54%; weight reduction 37% v 12%; anemia 33% v 44%; back pain 30% v 37%. Nine patients discontinued Xe due to AEs.
Based on this, the authors rightly conclude that the addition of Xe to Enza did not prolong PFS in mCRPC compared with Enza alone. There were no notable differences in PSA-related endpoints and CTC between arms.
However, it should be noted that these patients were very late in their treatment course and had already seen and failed both docetaxel and abiraterone. Hence, re-activating the AR sensitivity this late in the course may have doomed the study to failure. Perhaps in an earlier treatment time frame, different effects could have been seen.
Presented by: Syed A. Hussain, MD, Academic Unit of Oncology, Department of Oncology and Metabolism, University of Sheffield, Sheffield, England.
Co-Authors: Pablo Maroto, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Miguel Ángel Climent Instituto Valenciano de Oncología, Valencia, Spain; Diletta Bianchini, The Institute of Cancer Research and Royal Marsden, London, United Kingdom; Robert Hugh Jones, Velindre Cancer Centre and Cardiff University, Cardiff, United Kingdom; Chia-Chi Lin, Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan; Shian-Shiang Wang, Taichung Veterans General Hospital, Taichung, Taiwan; Emma Dean, The Christie NHS Foundation Trust, Manchester, United Kingdom; Kate Crossley, Boehringer Ingelheim Ltd, Bracknell, United Kingdom; Laura Schlieker, External Statistician on Behalf of Boehringer Ingelheim Pharma Gmbh & Co. KG, Staburo Gmbh & Co. KG., Munich, Germany; Thomas Bogenrieder, Boehringer Ingelheim RCV, Vienna, Austria, and Department of Urology, University Hospital Grosshadern, Ludwig-Maximilians-University, Munich, Germany; Johann S. De Bono Boehringer Ingelheim RCV, Vienna, Austria, and Department of Urology, University Hospital Grosshadern, Ludwig-Maximilians-University, Munich, Germany.
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology) at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA


