The PI3/AKT/mTOR pathway is frequently implicated in advanced prostate cancer, and PTEN loss occurs in up to 50% of all patients. Unfortunately, prior trials targeting the mTOR pathway have been largely unsuccessful, both in the single agent and combination setting.4 One theory is that you may need to block not only the mTOR pathway but also the PI3K pathway in combination with blocking AR signaling. In a PTEN deficient mouse model, a combination of enzalutamide plus a dual PI3K/mTOR1/2 inhibitor led to tumor regression by nearly 84%. Christopher Sweeney, MBBS et al describe the outcomes here of enzalutamide with LY or placebo in patients with metastatic castration-resistant prostate cancer (mCRPC) after progression on abiraterone.
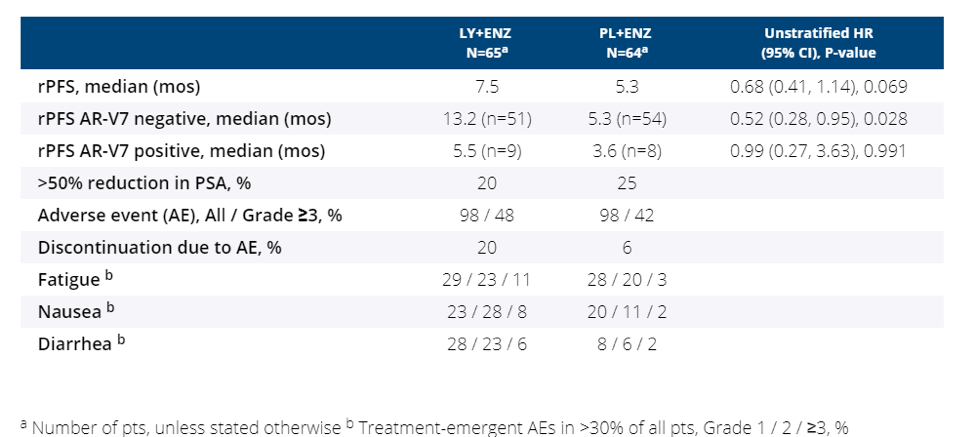
This abstract provides phase 1b data on 13 patients who have received LY+enzalutamide and 129 patients who were randomized in the phase II setting to LY+enzalutamide vs placebo+enzalutamide. All patients had mCRPC which had already progressed on abiraterone. The primary objective was progression-free survival (PFS) defined by PCWG2 criteria. For all patients, there was no significant difference between the LY group and placebo group with respect to progression-free survival (7.5 months vs 5.3 months, HR 0.68, p=0.069). However, for patients who were AR-V7 negative, the median radiographic progression-free survival (rPFS) was 13.2 months for the LY group and 5.3 for the placebo group (HR 0.52, p=0.028). For the AR-V7 positive group, there was no difference in rPFS. 20% of patients discontinued therapy in the LY group compared to 6% in the placebo group. The most common toxicities for the LY group was fatigue, nausea, and diarrhea.
Presented by: Christopher Sweeney, MBBS, Medical Oncologist, Medical Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Jason Zhu, MD, Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Smith MC, Mader MM, Cook JA, et al. Characterization of LY3023414, a novel PI3K/mTOR dual inhibitor eliciting transient target modulation to impede tumor growth. Molecular cancer therapeutics 2016;15:2344-56.
- Moore KN, Varghese AM, Hyman DM, et al. A phase I, first-in-human dose study of the dual PI3K/mTOR inhibitor LY3023414 (LY) in patients (pts) with advanced cancer. American Society of Clinical Oncology; 2015.
- Varghese AM, Moore KN, Hamilton EP, et al. Safety and tolerability of the dual PI3K/mTOR inhibitor LY3023414 in combination with fulvestrant in treatment-refractory advanced breast cancer patients. American Society of Clinical Oncology; 2017.
- Statz CM, Patterson SE, Mockus SM. mTOR inhibitors in castration-resistant prostate cancer: a systematic review. Targeted oncology 2017;12:47-59.


