The results demonstrated that apalutamide, in addition to ongoing ADT, prolonged metastasis-free survival (MFS) by more than two years, reduced the risk of symptomatic progression by 55%, and increased secondary progression-free survival, which was defined as the time from randomization to disease progression on first subsequent anticancer therapy, or death.
Figure 1 – SPARTAN study design:
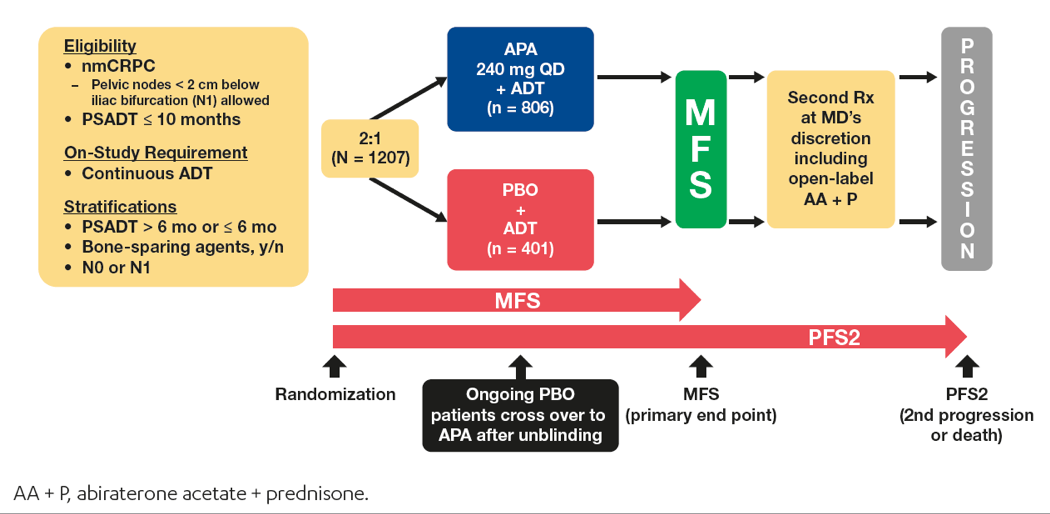
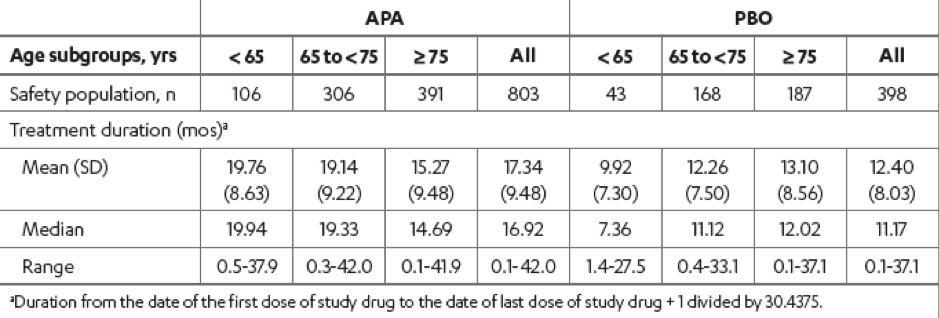
Among the 1207 randomized patients enrolled in the SPARTAN study, 149 (12.3%) were aged < 65 years, 476 (39.4%) were aged 65 to 75 years, and 582 (48.2%) were aged ≥ 75 years. The baseline characteristics of the patients in the study were similar with regards to age, although the ECOG performance status 1 vs. 0 increased with age (Table 1). The treatment duration stratified by age subgroup and treatment group is shown in Table 2.
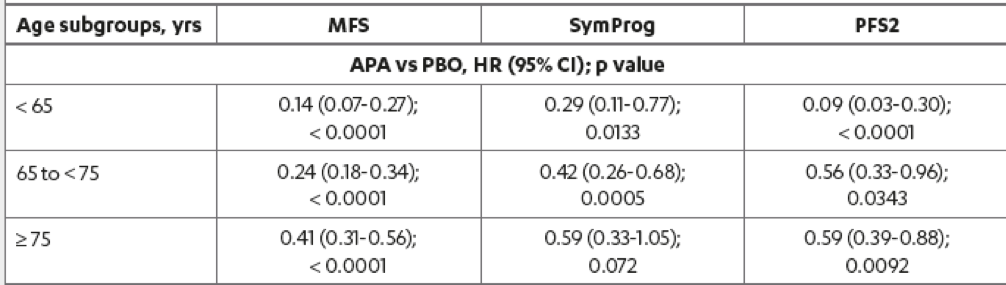
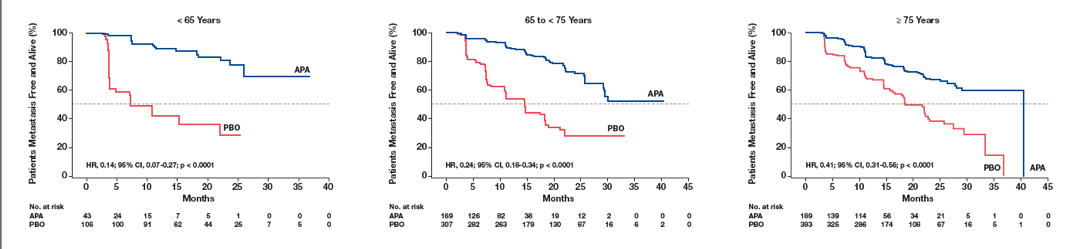
The results demonstrated that the MFS benefit with apalutamide was highly significant for all age subgroups (Table 3). In patients older than 75, aged between 65 and 74, and younger than 64 years, the MFS risk with Apalutamide compared to placebo was reduced by 59%, 76%, and 86%, respectively. Risk of secondary progression-free survival with apalutamide compared to placebo was reduced across all age subgroups. Secondary progression-free survival in patients younger than 65, 65-74, and ≥ 75 years were improved with apalutamide by 91%, 44%, and 41%, respectively.
The risk of symptomatic progression was reduced with apalutamide compared to placebo for all age subgroups, with the magnitude of MFS benefit with apalutamide being the greatest in the youngest age group (Figure 2).
Table 1 – Baseline patient and disease characteristics by age subgroups within treatment groups: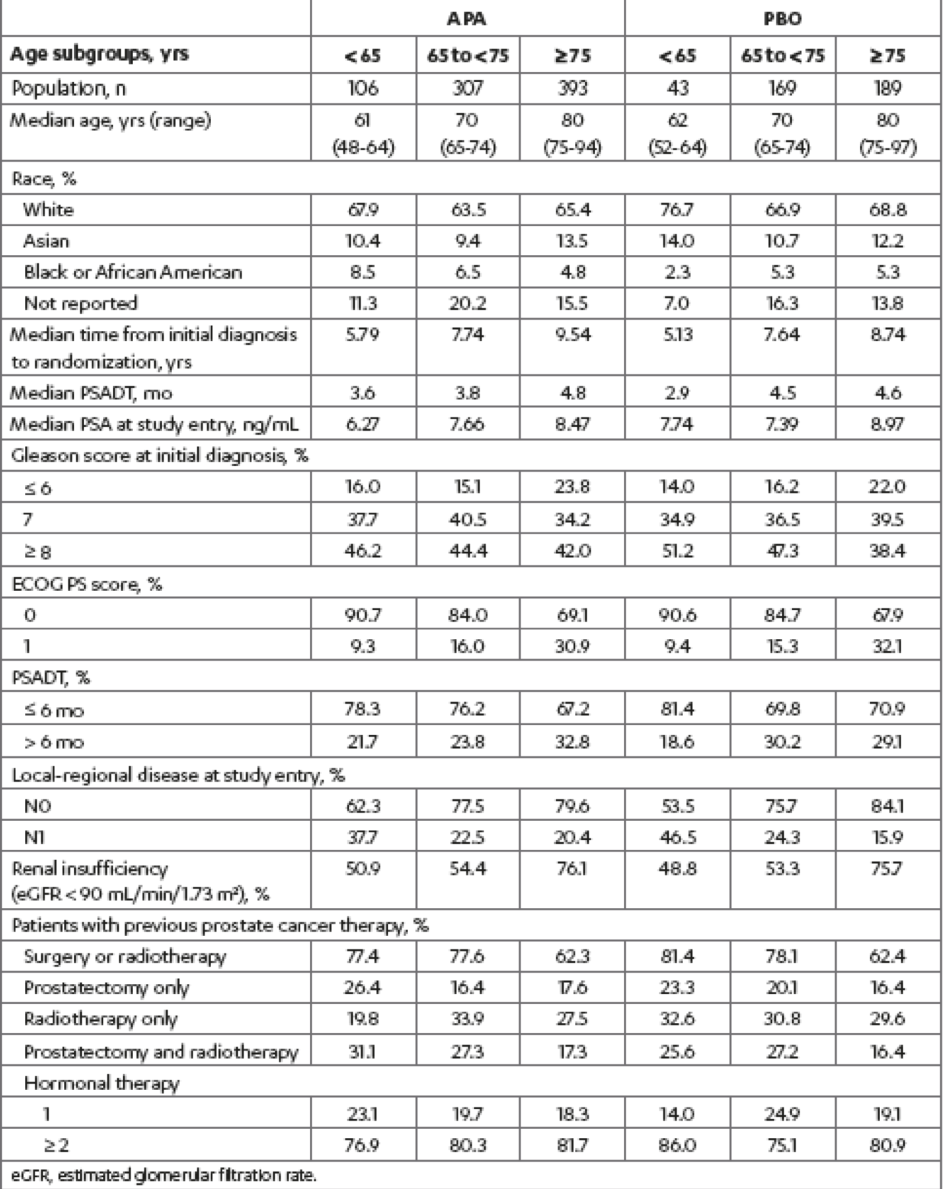

Table 2 – Treatment duration:
Lastly, there was a similar increase in the incidence of therapy-emergent adverse events with age in both treatment arms that remained consistently higher with apalutamide (Table 4). The incidence of grade 3/4 treatment-emergent adverse events in patients older than 75 compared to those younger than 65 was 50% vs. 37%, and 37% vs. 28% in the apalutamide and placebo arms, respectively. The rates of falls and fractures were highest in the >75 years age subgroup in both treatment groups.
Table 4 – Treatment-emergent adverse events:
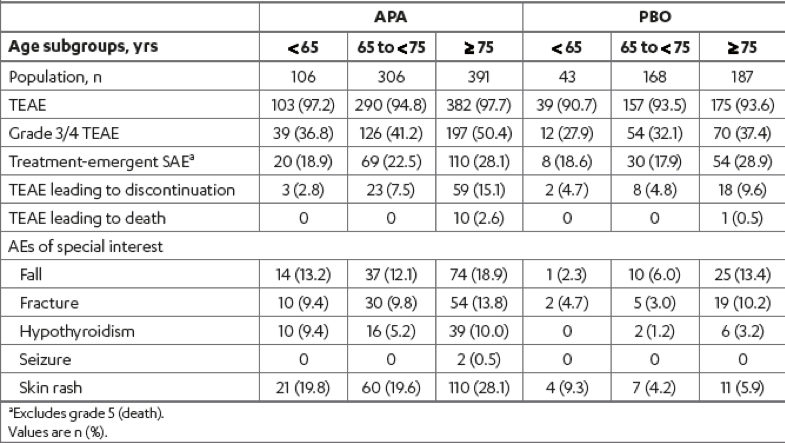
Generally, the adverse events increased with age in both treatment groups.
Regardless of age, SPARTAN patients with high-risk nmCRPC who received apalutamide had significantly better treatment outcomes than placebo-treated patients. Lastly, clinicians should counsel older patients on their increased risk of adverse events associated with apalutamide.
Presented by: Julie Nicole Graff, MD - Knight Cancer Institute, Oregon Health & Science University, Portland, OR
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Clegg NJ, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494-1503
- Smith MR, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918-2925.
- Scosyrev E, et al. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062-3070.
- Launay-Vacher V, et al. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol. 2007;18:1314-1321.
- Lichtman SM, et al. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14-34.


