In this study, men with mHSPC were randomly assigned 1:1 to receive testosterone suppression plus either enzalutamide or a non-steroidal anti-androgen. Randomization was stratified by:
- Volume of disease (high vs low, according to CHAARTED)
- Planned early DOC
- Planned anti-resorptive therapy
- Comorbidity score (ACE-27)
- Study site
Accrual of 1,100 men provided 80% power to detect a 25% reduction in the hazard of death (HR 0.75) with up to four interim analyses, the first planned to occur after 235 deaths. Subgroup analyses to assess possible modulation of the treatment effect were specified a priori and included planned early docetaxel (yes vs no) and volume of disease (high vs low). The ENZAMET trial schema is as follows:
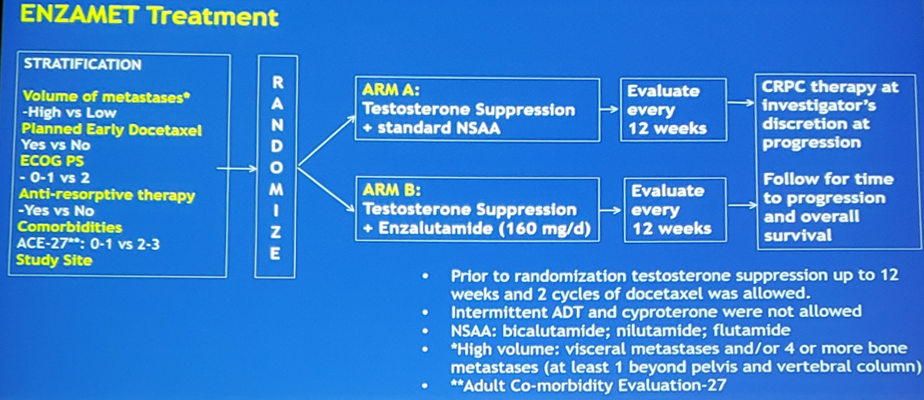
ENZAMET randomly assigned 1,125 patients from March 31, 2014 to March 24, 2017: 562 in the non-steroidal anti-androgen and 563 in the enzalutamide arm. The treatment groups were well balanced for all important baseline factors. In the non-steroidal anti-androgen arm 44% of patients were planned for early docetaxel, compared to 45% in the enzalutamide arm; 53% of patients in the non-steroidal anti-androgen arm were high volume metastatic burden, compared to 52% in the enzalutamide arm. Criteria for early reporting were met at the first interim analysis (February 28, 2019) after a median follow-up of 33 months – 143 deaths in the non-steroidal anti-androgen arm compared to 102 deaths in the enzalutamide arm.
Overall survival was significantly prolonged in the enzalutamide arm compared to the non-steroidal anti-androgen arm (HR 0.67, 95% CI 0.52-0.86):

Furthermore, time to PSA rise, clinical progression or death (HR 0.39, 95%CI 0.33-0.47) and time to clinical progression (HR 0.40, 95%CI 0.33-0.49) both favored enzalutamide:

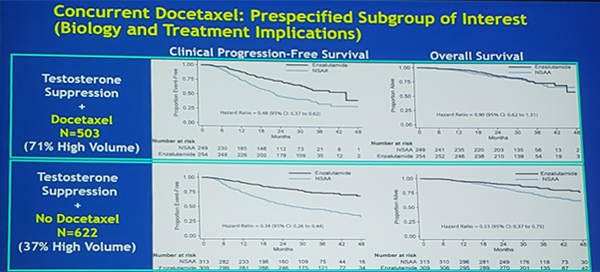
In the prespecified subgroup analysis of docetaxel (yes vs no), enzalutamide significantly improved time to clinical progression among men receiving docetaxel (HR 0.48, 95%CI 0.37-0.62), but did not improve OS (HR 0.90, 95%CI 0.62-1.31). In men not receiving docetaxel, enzalutamide improved clinical progression (HR 0.34, 95%CI 0.26-0.44) and OS (HR 0.53, 95%CI 0.37-0.75):
The 3-year OS point-estimates in predefined subgroups are as follows:

At 3 years, 36% of patients receiving non-steroidal anti-androgen compared to 64% of men receiving enzalutamide were still on their assigned study treatment. Serious adverse events (regardless of attribution) within 30 days of study treatment occurred in 42% in the enzalutamide arm, compared to 34% non-steroidal anti-androgen arm. There were 67% of patients in the enzalutamide arm that received one or more life prolonging CRPC therapies, compared to 85% in the non-steroidal anti-androgen arm, thus the OS benefits noted in this trial are unlikely to be secondary to discrepancies in subsequent therapy.
Dr. Sweeney concluded by noting that early enzalutamide improved time to progression and overall survival when added to standard mHSPC therapy (testosterone suppression +/- docetaxel). His final clinical interpretations are as follows:
- Enzalutamide added to testosterone suppression represents an appropriate option for men with metastatic prostate cancer commencing testosterone suppression
- There is a clear benefit for patients with low and high volume metastatic disease, including delays in progression and improvement in overall survival, however with more toxicity in the enzalutamide arm and more docetaxel-related toxicity with the addition of docetaxel
- For patients that are candidates for docetaxel when starting testosterone suppression, quality of life analyses and longer follow-up are needed to determine whether the delay in progression with concurrent enzalutamide results in a meaningful clinical benefit and/or is compounded by CRPC therapy and augments survival beyond 3 years
Clinical trial information: NCT02446405
Presented by: Christopher Sweeney, MBBS, Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, United States
Co-Authors: Andrew James Martin, Robert Richard Zielinski, Alastair Thomson, Thean Hsiang Tan, Shahneen Kaur Sandhu, M. Neil Reaume, David William Pook, Francis Parnis, Scott A. North, Ray McDermott, John McCaffrey, Gavin M. Marx, Nicola Jane Lawrence, Lisa Horvath, Mark Frydenberg, Simon Chowdhury, Kim N. Chi, Martin R. Stockler, Ian D. Davis; Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, MA; NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia; Central West Cancer Services, Orange, Australia; Royal Cornwall Hospital, Truro, United Kingdom; RAH Cancer Centre, Adelaide, Australia; Peter MacCallum Cancer Centre, The University of Melbourne, Melbourne, Australia; Ottawa Hospital Cancer Centre, Ottawa, ON, Canada; Department of Medical Oncology, Monash Health, Melbourne, VIC, Australia; University of Adelaide, Adelaide, Australia; University of Alberta Cross Cancer Institute, Edmonton, AB, Canada; Adelaide and Meath Hospital, Dublin, Ireland; Cancer Trials Ireland, Dublin, Ireland; University of Sydney Adventist Hospital, Sydney, Australia; Auckland City Hospital, Auckland, New Zealand; Sydney Cancer Centre, Sydney, Australia; Monash University, Clayton, Australia; Guy’s, King’s and St. Thomas’ Hospitals, and Sarah Cannon Research Institute, London, United Kingdom; BC Cancer, Vancouver, BC, Canada; NHMRC Clinical Trials Centre, The University of Sydney, Sydney, Australia; Monash University Eastern Health Clinical School, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. 2016;387(10024):1163-1177.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. Phase 3 study of androgen deprivation therapy (ADT) with enzalutamide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC): The ARCHES trial. J Clin Oncol 37, 2019 (suppl 7S; abstr 687).
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019 May 31 [Epub ahead of print].


