- Olaparib
- Docetaxel
- Enzalutamide
- Abiraterone

This abstract provides data on 72 patients who had progressed on either abiraterone or enzalutamide prior to treatment with chemotherapy. Patients received docetaxel 75 mg/m2 with pembrolizumab 200 mg IV every 3 weeks. Baseline characteristics shown below. The median age of this cohort was 68, 50% of patients had measurable disease, and 36% of patients had visceral metastases.

Almost all patients had some form of treatment-related adverse event (TRAEs), including alopecia (43%), fatigue (30%), and diarrhea (39%). Two patients had immune-related pneumonitis leading to death. The most commune immune-mediated adverse events (AEs) were colitis (10%) and infusion-related reactions (11%).

In terms of efficacy, the objective response rate for patients with measurable disease was 14%. 31% of all patients had a PS50. This is actually lower than the response rate in TAX327, the registrational trial for docetaxel which had a 45-48% PSA50 in the docetaxel arm (of note, patients in TAX327 were treatment naïve unlike the patients in this study who had either enza/abi so a very different patient population). The median radiographic progression-free survival (PFS) was 8 months and the median overall survival has not yet been reached in this study.

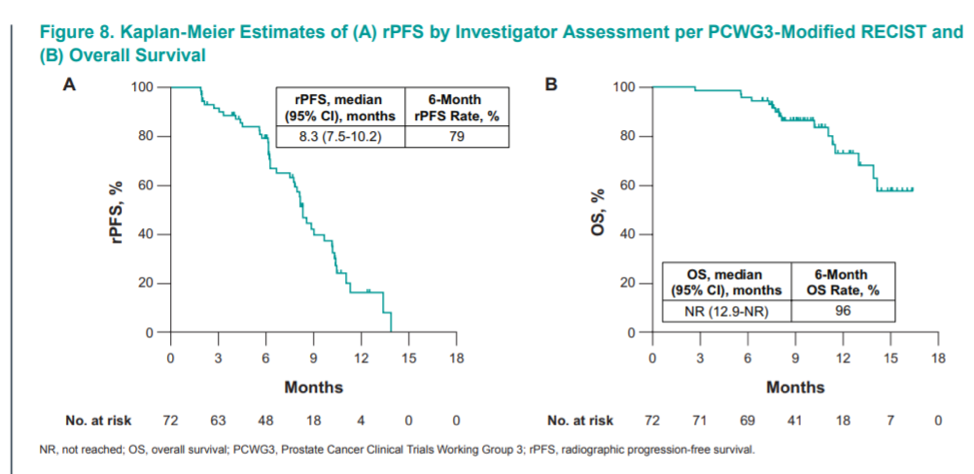
The combination of docetaxel and pembrolizumab is active 20-30% of patients with progressive disease after enzalutamide or abiraterone. However, this combination of therapy may not be appropriate for all patients given the toxicity of combination therapy and there were two treatment-related deaths due to pneumonitis. Additional biomarker work is necessary to determine which patients benefit best from immunotherapy in this setting. Additionally, future work should include exploratory biomarker work for toxicity, especially predictive markers for serious colitis and pneumonitis. A randomized phase three trial is now exploring docetaxel and prednisone in with and without pembrolizumab for mCRPC (KEYNOTE 921).
Presented by: Christophe Massard, MD, Ph.D., Medical Oncologist, Chair of Drug Development Department (DITEP), Gustave Roussy, Villejuif, France
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL US
References:
- De Bono JS, Goh JC, Ojamaa K, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2018.
- Hansen A, Massard C, Ott P, et al. Pembrolizumab for patients with advanced prostate adenocarcinoma: preliminary results from the KEYNOTE-028 study. Annals of Oncology 2016;27.


