
This abstract provides data on 1,125 patients who were randomly assigned to either ENZA or NSAA. The patient arms were well balanced and stratified disease volume based on CHAARTED criteria (Low volume = less than 4 bone metastases, high volume = 4 or more bone mets with one outside the spine/pelvis and or visceral metastases, age, comorbidities, planned early docetaxel, and study site. The primary end point was overall survival, and secondary endpoints included PSA progression and clinical progression-free survival, in addition to several correlative studies. Eligibility criteria are shown below.

Baseline characteristics are shown below. Patients were well balanced by age, region, and performance status.

Volume status was well matched as well. Early docetaxel occurred in 44-45% of all patients. The volume of metastases was similar in both arms and planned skeletal-related bone therapy was similar.

In terms of the primary endpoint, overall survival was significantly improved with enzalutamide over NSAA, with an HR of 0.67 (CI .52-.86, p=0.002), with 80% of patients alive at 3 years with enzalutamide and 72% with NSAA.

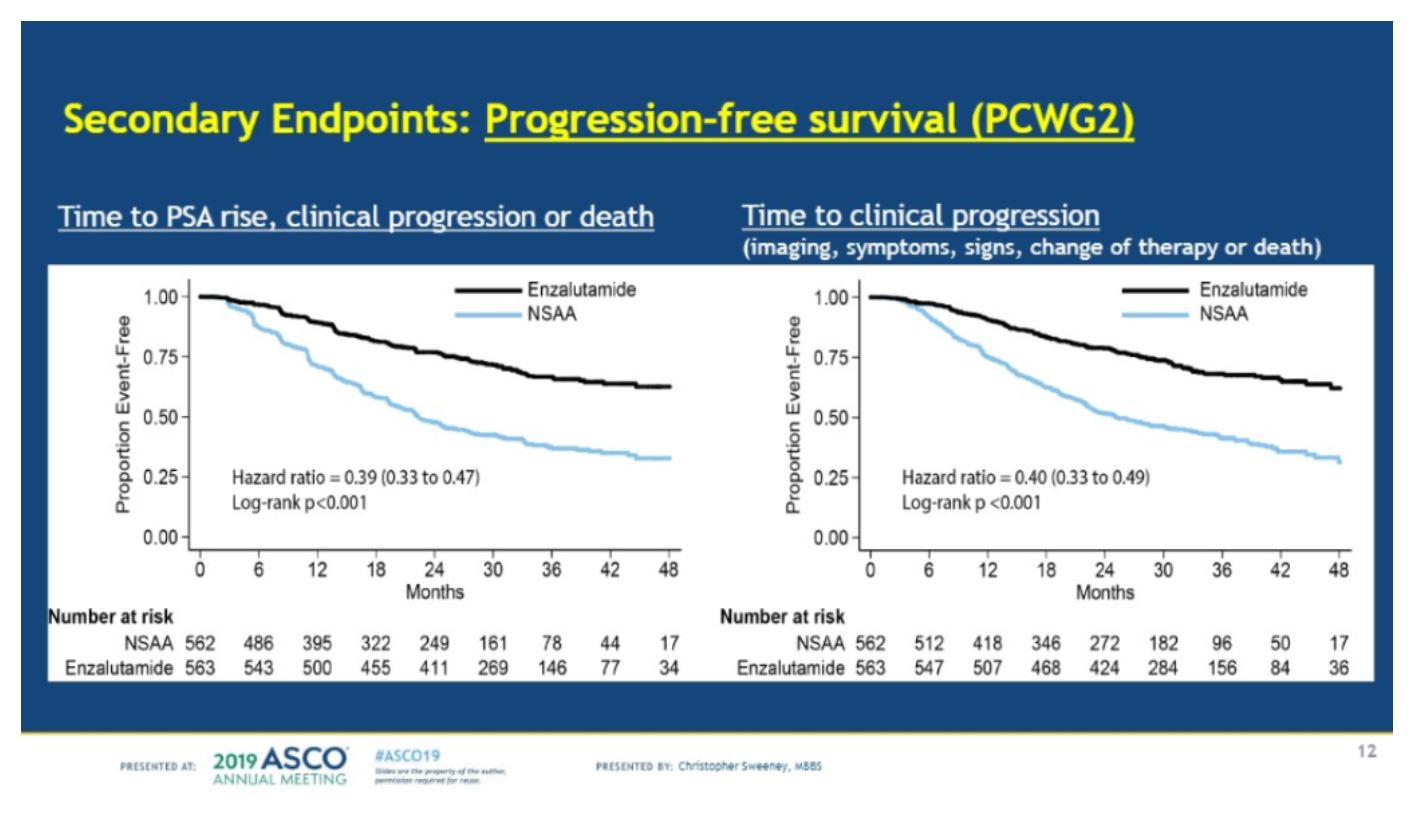
Secondary endpoints showed that enzalutamide was superior for time to PSA rise, clinical progression or death (HR=0.39) and time to clinical progression (HR=0.40).
In terms of concurrent docetaxel, there was no difference in overall survival between the two arms. However, there was a benefit in clinical progression-free survival (HR=0.48).

In the following discussion by Tanya Dorff, MD, she notes that there are several significant factors (cost, length of treatment, toxicity, and volume status) to consider when choosing amongst the collection of agents now approved for mHSPC.

In terms of toxicity, the serious AE rate was similar between NSAA and enzalutamide. Notable differences included greater grade 2/3 fatigue, syncope, and seizures, all known side effects of enzalutamide and consistent with prior studies.

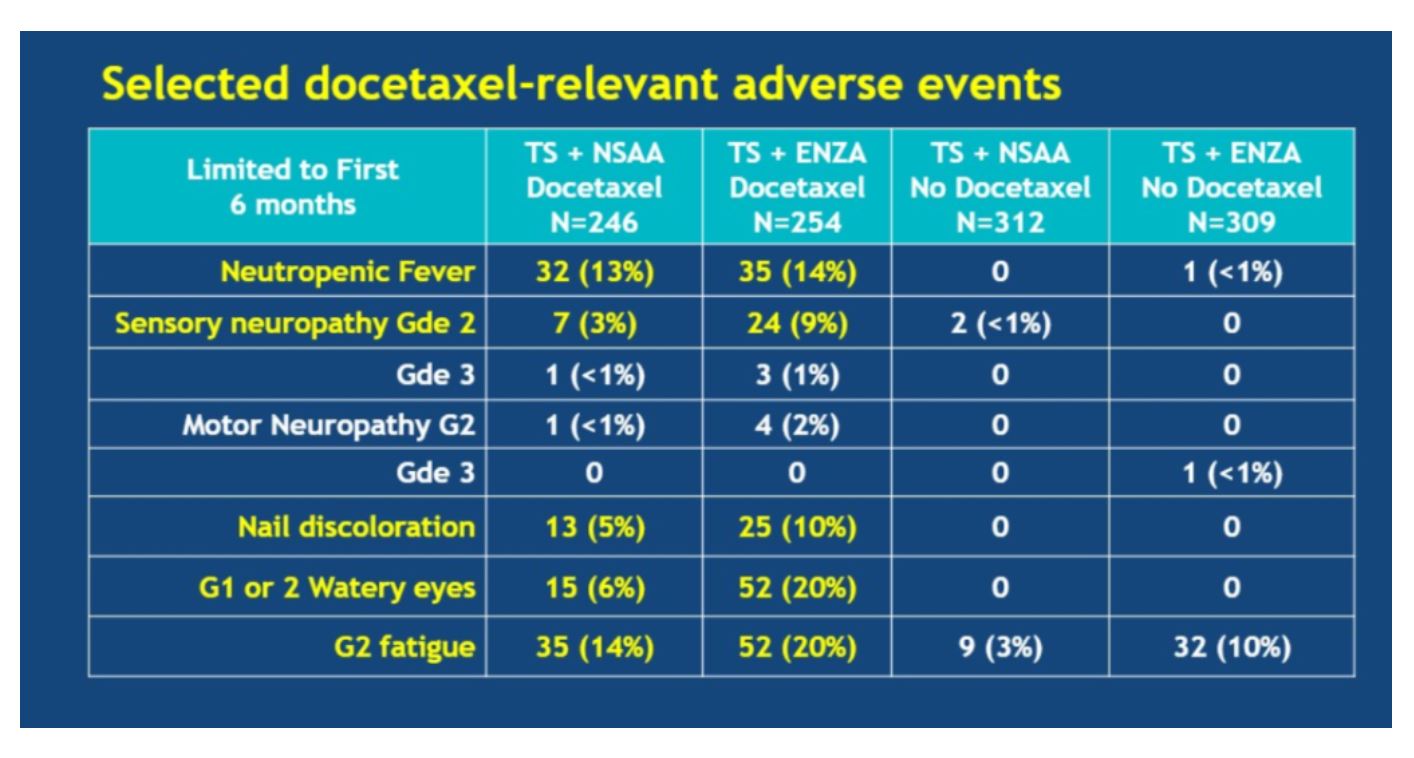
When enzalutamide was added to docetaxel, certain adverse events (AEs) became more common than with docetaxel alone, including grade 2 sensory neuropathy, nail discoloration, grade 1/2 watery eyes, and grade 2 fatigue.
Following Dr. Sweeney’s presentation, ENZAMET was published in the New England Journal of Medicine
Clinical trial information: NCT02446405
Presented By: Christopher Sweeney, MBBS, Medical Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Kyriakopoulos CE, Chen Y-H, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. Journal of Clinical Oncology 2018;36:1080.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 2017;377:338-51.



