This was a randomized open-label study comparing the one-year incidence of major cardiovascular and cerebrovascular events in prostate-cancer patients with pre-existing cardiovascular disease commencing on GnRH-agonists or antagonists. Patients were followed every 3 months for the development of major cardiovascular and cerebrovascular events defined as either death, myocardial infarction, cerebrovascular event, or percutaneous coronary intervention. Endothelial function was measured by EndoPAT200. Serum levels of N-terminal pro-B-type natriuretic peptide were analyzed at baseline, 3-, 6- and 12-months.
There were 80 patients enrolled: 41 randomized to GnRH-antagonist and 39 to GnRH-agonist. Patients in both arms had similar median age (72 vs 71 years), baseline cardiovascular and prostate-cancer characteristics. During follow-up, 15 patients developed a new cardiovascular event. Of these, nine patients developed major cardiovascular and cerebrovascular events (two deaths, one myocardial infarction, two cerebrovascular events, and four percutaneous-coronary interventions). Twenty percent (n=8) of patients randomized to GnRH-agonists had a major cardiovascular and cerebrovascular event compared to 3% (n=1) randomized to antagonists (log-rank p=0.013).
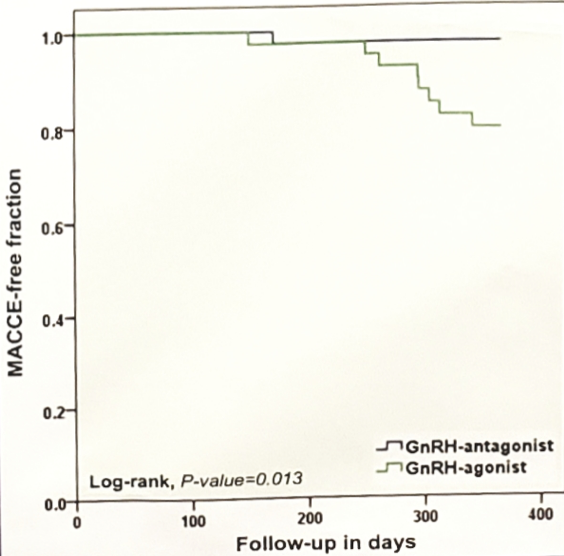
Dr. Margel concluded this study by noting that this is the first prospective study to test cardiovascular outcome among prostate-cancer patients receiving ADT. In patients with pre-existing cardiovascular disease, GnRH-antagonists was associated with the development of fewer cardiovascular events compared to GnRH-agonists.
Clinical trial information: NCT02475057
Presented by: David Margel, MD, PhD, Rabin Medical Center, Petah Tikva, Israel
Co-Authors: Avivit Peer, Yaara Ber, Sivan Sela, Liat Shavit Grievink, Tzlil Tabachnik, Guy Witberg, Jack Baniel, Daniel Kedar, Wilhelmina CM Duivenvoorden, Eli Rosenbaum, Jehonathan H Pinthus; Rabin Medical Center, Petah Tikva, Israel; Rambam Health Care Campus, Haifa, Israel; Rabin Medical Center, Prtah Tikva, Israel; Tel Aviv University, Tel Aviv, Israel; Rabin Medical Center, Petach Tikvah, Israel; McMaster University, Hamilton, ON, Canada; Davidoff Cancer Center, Petah Tikva, Israel
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA


