Chicago, IL (UroToday.com) Sipuleucel T is an active cellular immunotherapy which consists of autologous peripheral blood mononuclear cells which have been activated ex vivo with PA2024, a recombinant fusion protein consisting of prostate antigen and prostatic acid phosphatase.1 In IMPACT, the registration trial for sipuleucel T, treatment with sip T resulted in a 22% reduction in the risk of death compared with placebo (hazard ratio (HR)=0.78; 95% confidence interval [CI], 0.61 to 0.98; P=0.03) and improved median overall survival by 4.1 months.2 In the original study, the patient population was 90% Caucasian, with only 6.7% African American patients. This abstract provides additional data regarding the effect of sip T on African American patients based on the PROCEED registry, a large real-world registry of patients receiving sip T.
This abstract provides data on 221 African American (AA) patients and 1649 Caucasian (CAU) patients who received sipuleucel-T. In terms of all patients, the median overall survival (OS) for AA patients was 35.2 months and the median OS for CAU patients was 25.8 months.
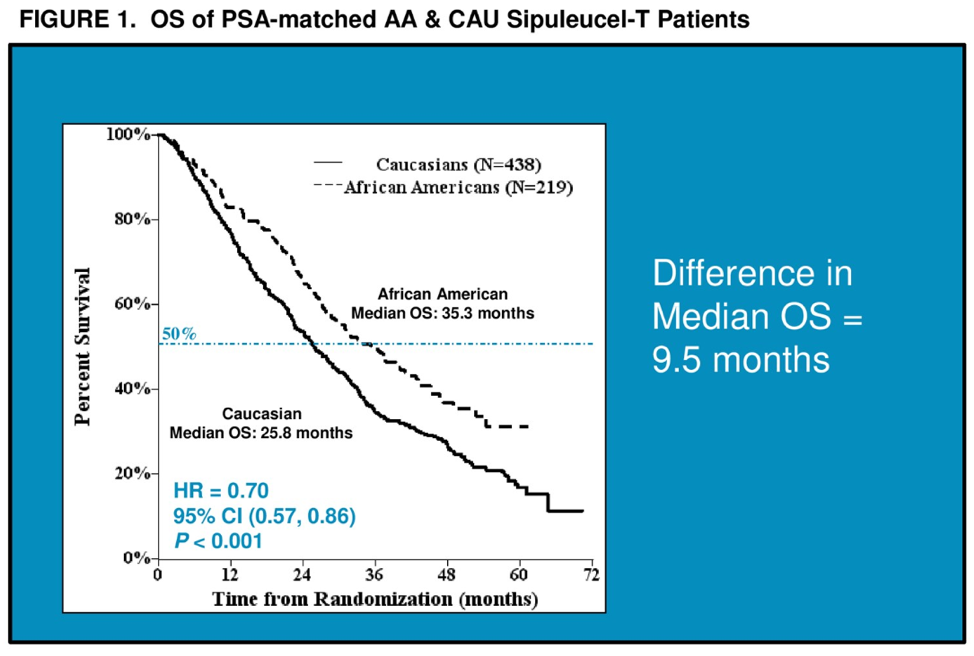
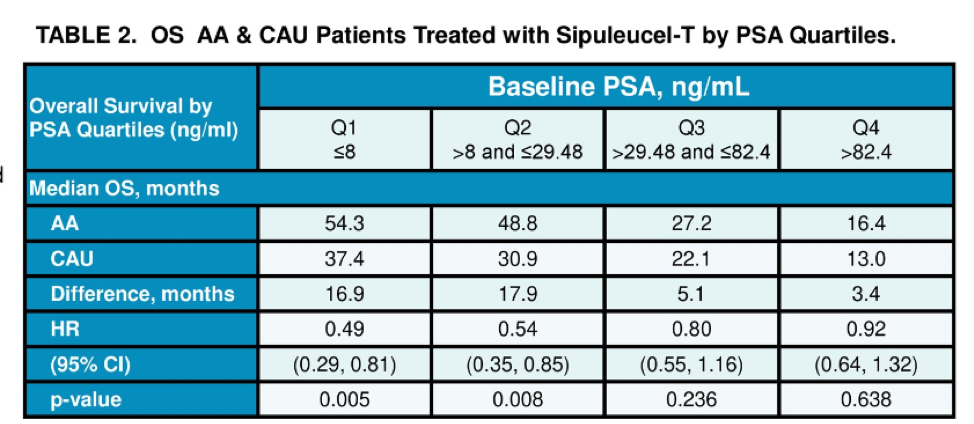
When patients were matched for prostate-specific antigen (PSA), prognostic variable for overall survival after treatment with sip T, AAs continued to maintain an overall survival advantage with a median OS of 35.3 months compared to 25.8 months for CAU patients. The median baseline PSA prior to sip T was 29.48 ng/mL. Among patients with PSAs less than 29, the median OS was 54.3 months in the AA cohort and 33.4 months in the CAU cohort. After adjusting for other known prognostic factors, AA race was associated with prolonged OS benefit compared with CAU, HR=0.60, 95% CI 0.48–0.74; p < 0.001.


Presented by: A. Oliver Sartor, MD, Professor of Medicine, Medical Director, Tulane Cancer Center, New Orleans, LA
Written by: Jason Zhu, MD, Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New England Journal of Medicine 2010;363:411-22.
- Abida W, Armenia J, Gopalan A, et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precision Oncology 2017:1-16.


