Biomarkers for response to anti-PD-L1 therapy continue to be characterized. Analysis of pre-treatment biopsies from the IMVigor210 trial showed that response to atezolizumab was associated with a PD-L1 expression on tumor cells, a CD8+ T-effector phenotype RNA expression signature in tumors, and higher tumor mutational burden. Conversely, TGF-b gene signaling in the tumor stroma and exclusion of immune cells (immune excluded or immune desert) were associated with a lack of response. To explore the potential predictive role of these identified biomarkers, in this presentation, Dr. Matthew Galsky presented correlative molecular analysis of pre-treatment samples from patients treated on the IMvigor130 trial.
Of the 1,213 patients enrolled, RNA sequencing data was available from 928 pre-treatment tumor samples, DNA sequencing data was available from 1,006 samples, and 852 patients had tumor samples processed for both DNA and RNA sequencing.
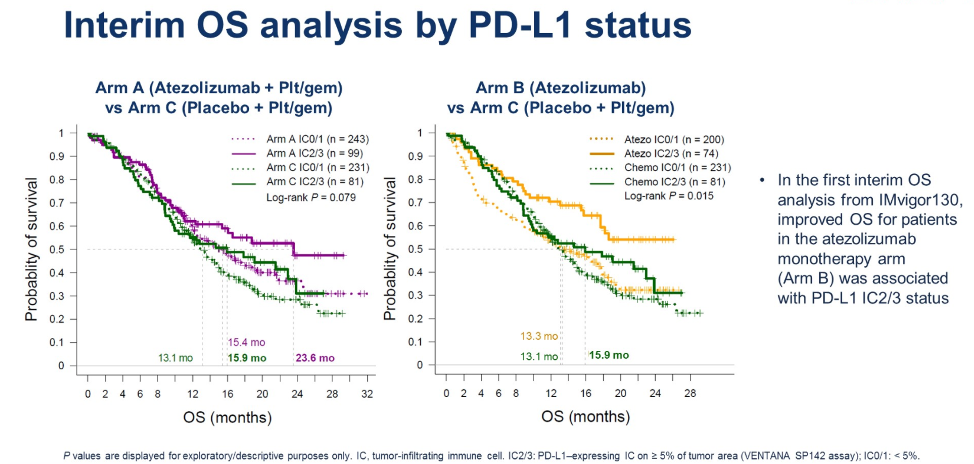
When stratifying by PD-L1 expression on tumor-infiltrating immune cells, patients with greater than 5% PD-L1 expression (IC2/3) appear to have numerically longer survival when treated with atezolizumab either alone or in combination with chemotherapy.
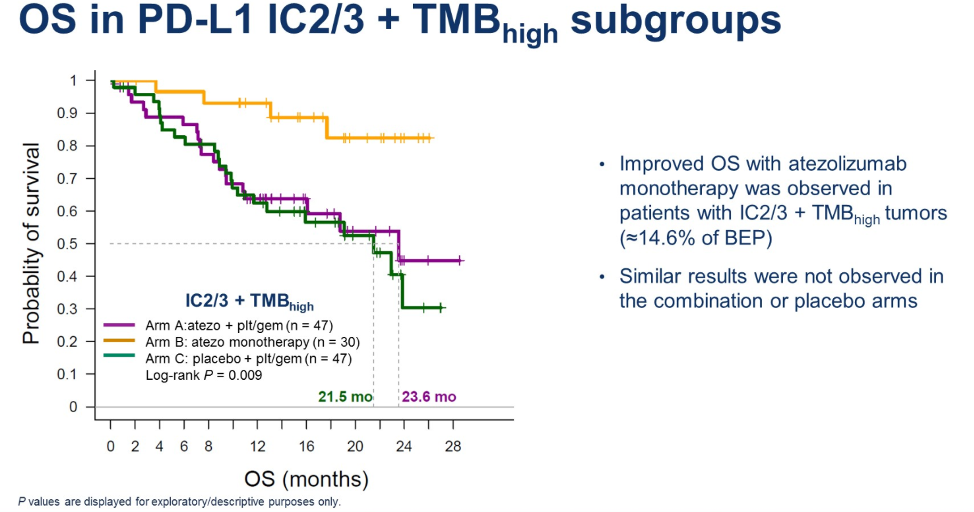
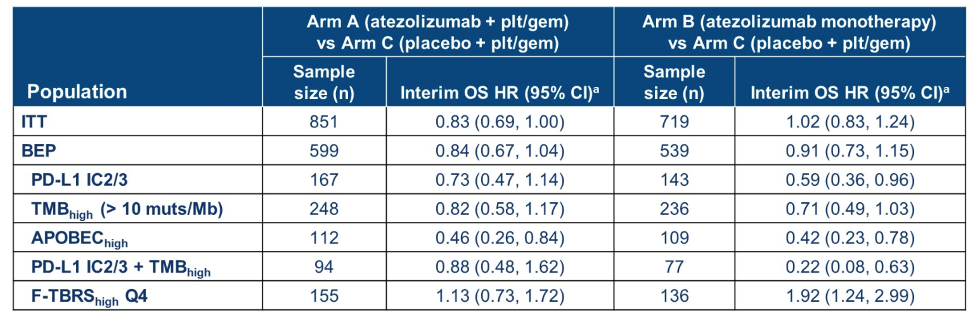
By DNA sequencing, higher tumor mutational burden (TMB) was associated with the APOBEC mutational signature, which is the predominant mutational signature present in urothelial carcinomas. In patients treated with atezolizumab monotherapy or in combination with chemotherapy, higher tumor mutational burden and the presence of the APOBEC mutational signature were associated with numerically improved overall survival. Almost 15% of evaluable patients were classified as high PD-L1 (greater than 5%) and high TMB (greater than 10 mutations/MB). In the atezolizumab monotherapy arm, these patients had a statistically significant longer overall survival.
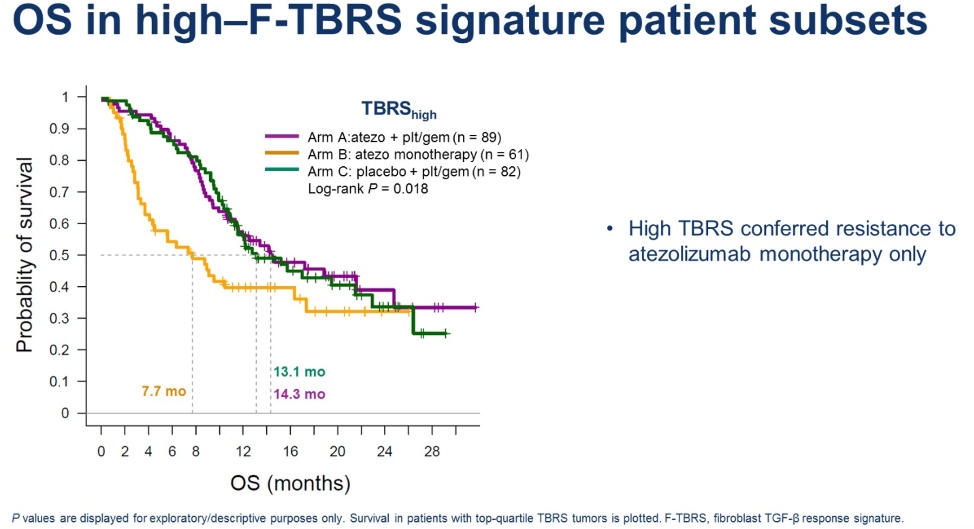
With regards to resistance, expression of a TGF-b response signature in tumor fibroblasts was associated with worse overall survival.
In summary, multiple biomarkers may help predict which patients with locally advanced or metastatic urothelial carcinoma derive greater benefit from upfront anti-PD-L1 therapy with atezolizumab.
Presented by: Matthew D. Galsky, MD, Professor of Medicine, Director of Genitourinary Medical Oncology, Director of the Novel Therapeutics Unit, Co-Director of the Center of Excellence for Bladder Cancer, Tisch Cancer Institute and Icahn School of Medicine, Mount Sinai, New York, New York
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, Boston, Massachusetts, at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020


