KEYNOTE-992 is a phase 3, global, multicenter, double-blind, placebo-controlled, randomized trial to evaluate the efficacy and safety of pembrolizumab + chemoradiotherapy versus placebo + chemoradiotherapy in patients with previously untreated muscle invasive bladder cancer. Adults (≥18 years) opting for bladder preservation with histologically confirmed cT2-T4a, nonmetastatic (N0M0) muscle invasive disease after maximal TURBT are eligible. An estimated 636 patients will be randomly assigned 1:1 to receive chemoradiotherapy + either pembrolizumab 400 mg IV every 6 weeks or placebo.
The trial design for KEYNOTE-992 is as follows:
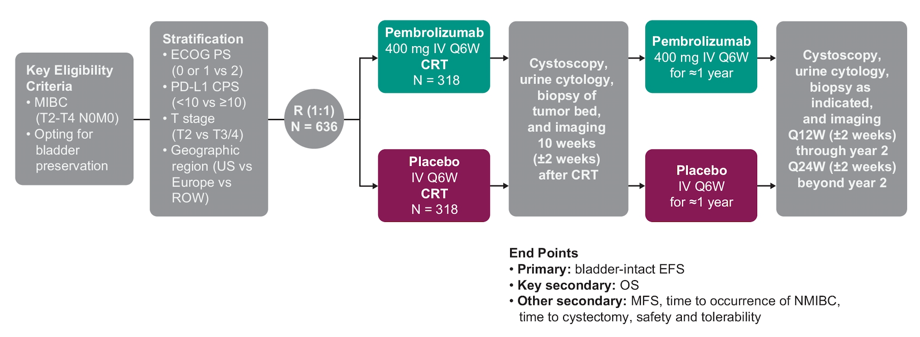
Treatment will continue with pembrolizumab or placebo every 6 weeks for up to nine doses. Chemoradiotherapy regimens will be decided by the investigator before randomization. Accepted radiotherapy regimens are conventional radiotherapy consisting of 64 Gy at 2 Gy/fraction over 6.5 weeks (whole bladder with or without pelvic nodes) or hypofractionated radiotherapy consisting of 55 Gy at 2.75 Gy/fraction over 4 weeks (whole bladder only). Accepted concurrent radiosensitizing chemotherapy regimens are cisplatin monotherapy (35 mg/m2 IV weekly), 5-fluorouracil (500 mg/m2 on days 1-5 and days 22-26) + mitomycin C (12 mg/m2 on day 1), or gemcitabine monotherapy (27 mg/m2 IV twice weekly). Randomization will be stratified by ECOG performance status (0/1 vs 2), PD-L1 combined positive score (< 10 vs ≥10), T stage (T2 vs T3/4), and geographic region (US vs Europe vs rest of world). Additionally, patients must provide tissue for biomarker analysis. Efficacy will be assessed by cystoscopy (± biopsy), CT or MRI (with blinded independent central review), and urine cytology at 10 weeks after chemoradiotherapy, then every 12 weeks up to the end of year 2, and then every 24 weeks thereafter. The primary endpoint is bladder-intact event-free survival, defined as time from randomization to residual/recurrent muscle invasive disease, nodal or distant metastasis, radical cystectomy, or death from any cause.
Secondary endpoints include:
- Overall survival
- Metastasis-free survival
- Time to occurrence of NMIBC
- Time to cystectomy
- Safety and tolerability
Clinical trial information: NCT04241185.
References:
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018 Dec 1;36(34):3353-3360.
- Balar AV, et al. J Clin Oncol 2019;37(suppl 7). Abstract 350.
- Weickhardt AJ, et al. J Clin Oncol 2020;38(suppl 6). Abstract 485.
Co-Authors: Nicholas D. James, Shahrokh F. Shariat, Neal D. Shore, Michiel Simon Van Der Heijden, Andrew James Weickhardt, Xiao Fang, James Luke Godwin, Ekta Kapadia, Jeff M. Michalski; Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom; Medical University of Vienna, Vienna, Austria; Carolina Urologic Research Center, Myrtle Beach, SC; The Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands; Olivia Newton-John Cancer Wellness & Research Centre, Heidelberg, VIC, Australia; Merck & Co., Inc., Kenilworth, NJ; Washington University in St. Louis School of Medicine, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.


