(UroToday.com) After frontline platinum chemotherapy for patients with metastatic bladder cancer, on progression, patients receive immune checkpoint inhibitors. Unfortunately, the majority of patients do not respond to single-agent immunotherapy. IMvigor211 (Atezolizumab) had an ORR of 23%, KEYNOTE045 (Pembrolizumab) had an ORR of 21%, and CheckMate275 (Nivolumab) had an ORR of 19.6%. Thus, several approaches are being studied to modulate and improve the efficacy of immunotherapy in the second line. This study evaluates combining cabozantinib, a multikinase inhibitor (MET, AXL, and VEGFR) with atezolizumab, for patients who had progressed on or after platinum-containing chemotherapy.

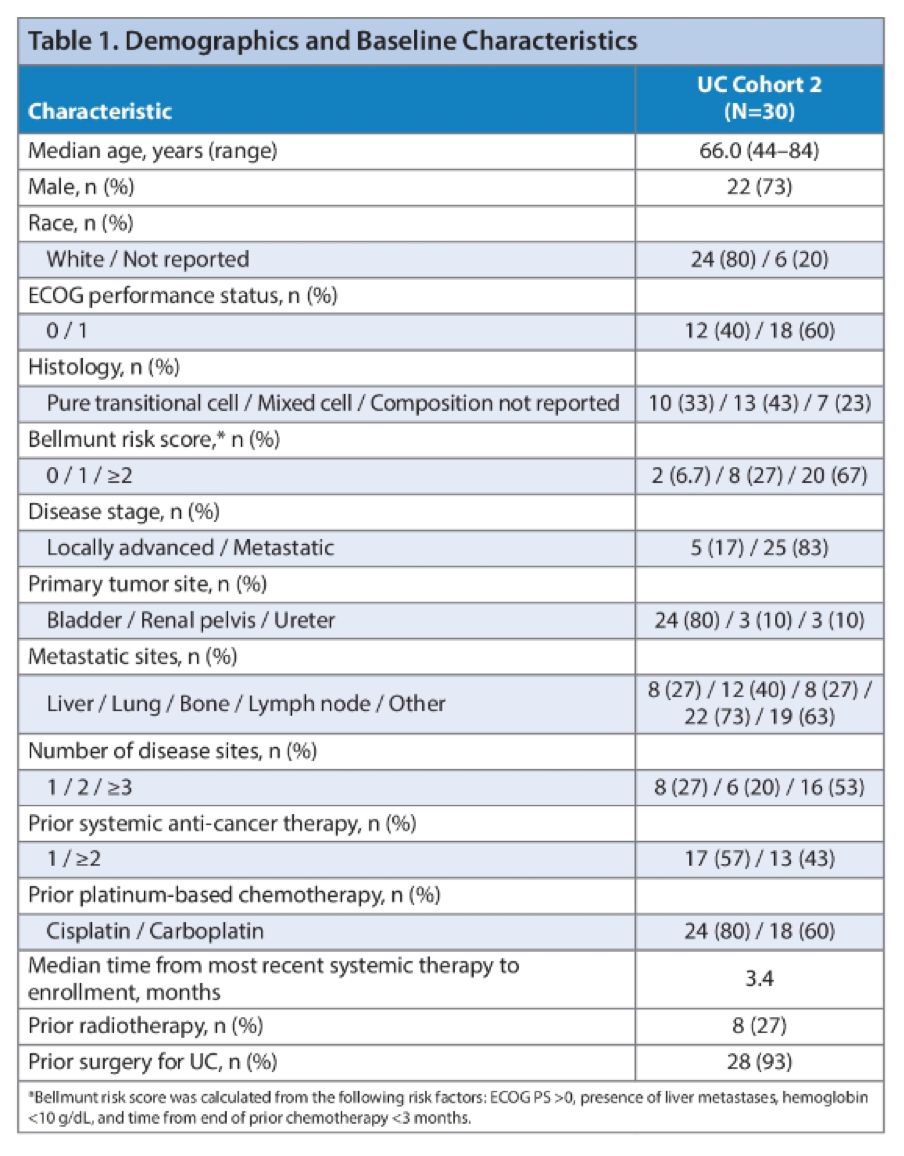
The study schema is shown above. 30 patients with advanced UC were enrolled with a median follow up of 16.5 months. 40% of patients had lung metastases and 27% of patients had liver metastases. Almost half the patients (47%) had progressed on 2 or more lines of systemic therapy. Of note, only 1/3 of patients had a pure urothelial carcinoma. 43% of patients had mixed histology and 23% were unreported. This was a high-risk population with 67% having a Bellmunt risk score of ≥2.
The primary endpoint was objective response rate (ORR), and secondary endpoints included safety, duration of response, PFS, and OS. The confirmed objective response was 27%, with 2 patients achieving a CR. The median duration of response has not been reached with one patient with an ongoing response at 15.6 months. The median progression-free survival was 5.4 months. 55% of patients had any reduction in target lesion size and preliminary biomarker work suggests that there was no association between PD-L1 expression and tumor response.
In terms of safety, grade 3/4 treatment-related adverse events (TRAEs) occurred in 57% of patients. The most common TRAEs were fatigue (37%), diarrhea (27%), decreased appetite (23%), increased transaminases (23%), and mucosal inflammation (20%).

Combination cabozantinib plus atezolizumab demonstrates clinical activity in patients with advanced urothelial carcinoma. It will be important to compare single-agent immunotherapy to combination approaches in the future to determine if there are benefits to overall survival and progression-free survival at the risk of increased toxicities.
Presented by: Sumanta Kumar Pal, MD, Associate Clinical Professor, Department of Medical Oncology & Therapeutics Research, Co-director, Kidney Cancer Program, Medical Oncologist, City of Hope, Duarte, CA
Written by: Jason Zhu, MD. Medical Oncologist, Division of Genitourinary Cancers, Levine Cancer Institute, Twitter: @TheRealJasonZhu, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
Related Content:
Read: Results from COSMIC-021 Trial Announced: Cabozantinib in Combination with Atezolizumab with Multiple Advanced Solid Tumor Types


