(UroToday.com) The oral abstract session for kidney and bladder cancer at the virtual ASCO 2020 annual meeting featured a discussant presentation by Daniel Heng, MD, MPH, FRCPC, discussing new and updated trials of single-agent and combination immunocheckpoint blockade. Immune checkpoint inhibitors in bladder cancer were first approved in the metastatic, post-platinum setting of which there are now five approved agents. Based on the success in the post-platinum metastatic setting, we have seen these therapies move up further in the disease process as summarized in the following figure:

Until the presentation of IMvigor 010 at ASCO 2020, we did not have data for immune checkpoint inhibitors in the adjuvant setting. IMVigor 010 enrolled patients with high-risk muscle-invasive urothelial carcinoma who underwent a radical cystectomy/nephroureterectomy with lymph node dissection within 14 weeks of enrollment. This included ypT2-T4a or ypN+ patients previously treated with neoadjuvant chemotherapy or pT3-pT4a or pN+ patients not treated with neoadjuvant chemotherapy. These patients were then randomized 1:1 to receive atezolizumab 1200 mg every three weeks vs observation, with a primary endpoint of disease-free survival and key secondary endpoints of overall survival. The IMvigor 010 study design is as follows:
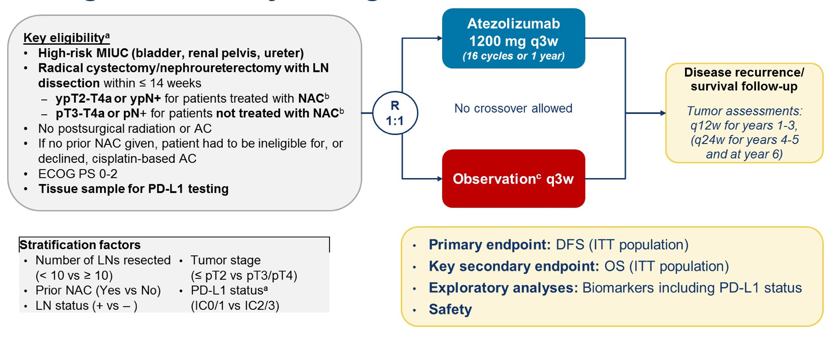
The primary outcome of disease-free survival in the ITT population was negative with a hazard ratio of 0.89 (95% CI 0.74-1.08) and an 18-month disease-free survival rate of 51% for atezolizumab versus 49% for the observation arm. Even in subgroup analyses by PD-L1 status, there was no benefit for atezolizumab: PD-L1 IC0/1 HR 0.81 (95% CI 0.63-1.05) and PD-L1 IC2/3 HR 1.01 (95% CI 0.75-1.35). Furthermore, there was no clinical subgroup that showed a benefit for adjuvant atezolizumab include disease site, pT2N0, pT3N0, and pT4N0. According to Dr. Heng, there is currently no role for adjuvant atezolizumab in muscle-invasive bladder cancer. Why there is a PD(L)-1 benefit in the metastatic and not in the adjuvant setting remains to be fully elucidated. Perhaps it is because gross tumor needs to be in situ to potentiate the immune response, or perhaps not all drug classes are equal. Furthermore, even though the trial was slightly more enriched for PDL1+ patients (first 119 patients), the trial is still negative, even in the subgroup of PDL1 IC2/3 patients. Only 47-48% of patients had neoadjuvant chemotherapy, and there was no benefit for atezolizumab in this group. Only 12-14% of patients had T4 disease, but this was the group with the lowest hazard ratio (HR 0.60, 95% CI 0.29-1.25). There are many ongoing phase I/II trials evaluating the role of neoadjuvant therapy for muscle-invasive bladder cancer as outlined in the table below:1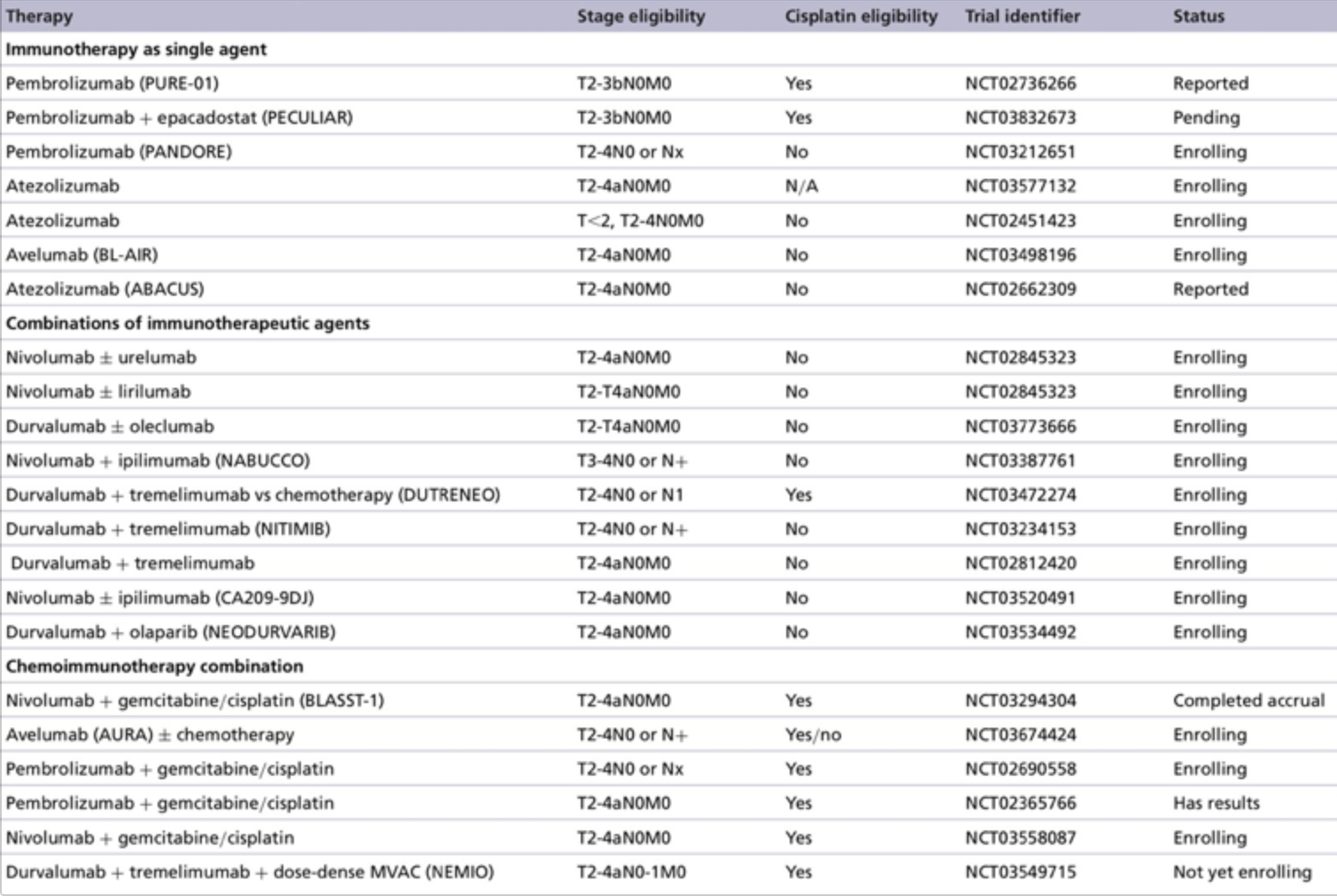
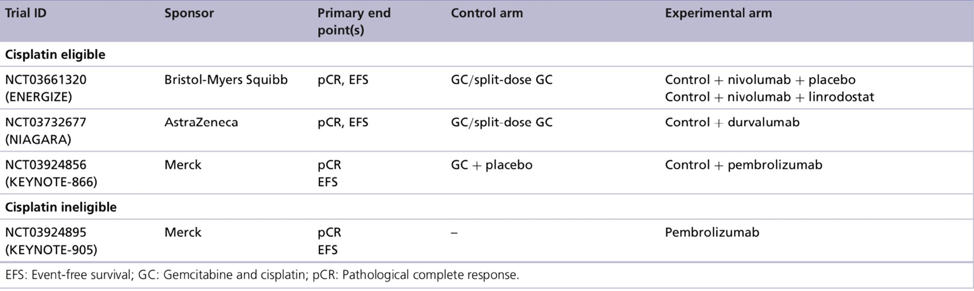
Additionally, there are several ongoing phase III trials in this disease space:
Dr. Heng then discussed the updated data from the KEYNOTE-426 study, a trial of newly diagnosed or recurrent stage IV clear cell renal cell carcinoma randomized 1:1 (n=861) to receive pembrolizumab 200 mg IV every three weeks + axitinib 5 mg BID vs sunitinib 50 mg orally daily for the first four weeks of each 6 week cycle.2 With a minimum of 23 months follow-up in the updated analysis, OS in the ITT population continued to favor the pembrolizumab + axitinib group with a hazard ratio of 0.68 (95% CI 0.55-0.85). Updated response rates show that pembrolizumab + axitinib complete response rate is now up to 9% compared to 6% in the original data reporting, with an overall response rate of 60%. Dr. Heng notes that it is tempting to compare objective response rates between the KEYNOTE-426 trial and the CheckMate-214 trial,3,4 but this must be taken into context given the differences in IMDC risk stratification of enrolled patients:

Furthermore, nivolumab + ipilimumab was inferior to sunitinib for objective response rate in favorable risk patients, thus it is only approved in intermediate/poor risk patients. When stratified by IMDC risk factors, regardless of number of risk factors, there is a stable response for nivolumab + ipilimumab at ~41%, compared to sunitinib which continues to lose efficacy with increasing IMDC risk factors.5
With regards to discussion points for KEYNOTE-426, Dr. Heng notes that it is important that the complete response rate has moved up to 8.8%, but it is still difficult to compare across trials as >30% of patients in this trial were IMDC favorable-risk disease (with an 11% complete response rate in this group). The progressive disease rate of only 11.3% is very attractive, as well as 94% of patients achieving some reduction in target lesions. Additionally, the depth of response is notable in that an 80% reduction compared to a complete response may produce similar overall survival benefit, although the follow-up is still short and we must assess durability of complete responses. Finally, the favorable risk disease OS data is not yet statistically significant and it may take a long time for the data to mature, given that the trial was not powered for assessing this subgroup. According to Dr. Heng, the primary endpoint was all-risk IMDC PFS/OS, thus pembrolizumab + axitinib should be given regardless of risk category. Dr. Heng’s preference is for nivolumab + ipilimumab for patients with IMDC poor/intermediate risk disease, those with no significant autoimmune history, patients with VEGF contraindications and those that he wants to use a CTLA4 now and a VEGF later. He chooses pembrolizumab + axitinib for patients with IMDC favorable/intermediate risk, those that would do better with less immunotherapy toxicity, those that can tolerate a VEGF inhibitor, and for those that will not tolerate progressive disease. Excitingly, more data is coming in the first-line setting for metastatic RCC as highlighted by the following figure: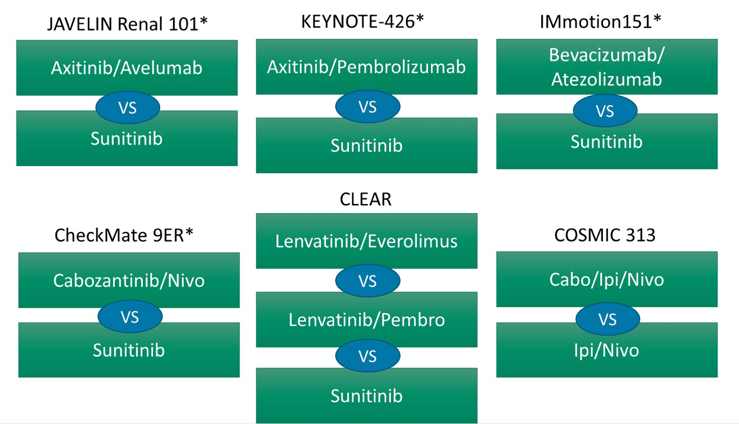
Presented by: Daniel Y. C. Heng, MD, MPH, FRCPC, Department of Medical Oncology, Tom Baker Cancer Center, University of Calgary, Calgary, Alberta, Canada
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
References:
- Sonpavde G, Necchi A, Gupta S, et al. ENERGIZE: A Phase III Study of neoadjuvant chemotherapy alone or with nivolumab with/without linrodostat mesylate for muscle-invasive bladder cancer. Future Oncol 2020 Jan;16(2):4359-4368.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378(14):1277-1290.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomized, controlled, phase 3 trial. Lancet Oncol 2019 Oct;20(10):1370-1385.
- Escudier B, Motzer RJ, Tannir NM, et al. Efficacy of Nivolumab plus Ipilimumab According to Number of IMDC Risk Factors in CheckMate 214. Eur Urol 2020 Apr;77(4):449-453.


