(UroToday.com) Advanced urothelial carcinoma resulted in over 200,000 deaths across the world in 2018. Though the majority of patients eligible for such therapy respond to platinum-based chemotherapy, disease progression occurs relatively quickly and a half or less of patients receive second-line treatment. Though PD-L1/PD-1 immune checkpoint blockade (ICB) agents are standard 2nd-line therapy for disease progression after platinum, not all patients receive this therapy and only a minority of patients have a durable clinical benefit. Avelumab, an antibody against PD-L1, is approved in the second-line post-platinum treatment setting.
In this presentation, the authors report results from a phase 3 randomized study for maintenance avelumab therapy in patients who derived disease response or stabilization from first-line platinum-based chemotherapy for advanced urothelial cancer. The study design is shown below. 
Patients had to have received at least 4 cycles of front-line chemotherapy and have had at minimum stable disease on therapy. Within ten weeks of completion of chemotherapy, they were randomized to best supportive care or avelumab maintenance therapy. There was no placebo infusion. Patients were stratified by best response to 1st line chemotherapy, as well as metastatic site. The primary endpoint of this study was overall survival in the entire intention-to-treat population as well as in the PD-L1 biomarker positive population. PD-L1 positivity was defined as shown in the image above. Crossover was not allowed.
Dr. Powles presented data from the planned interim analysis, which was triggered after 324 events (76.2% of information fraction) occurred in the overall population and 143 events (65.3% information fraction) in the PD-L1+ population.
Patient baseline characteristics were well-balanced between groups, though the PD-L1 positive group had higher rates of non-visceral metastases.
The data cutoff for this analysis occurred in late 2019, with 19.6-month median follow-up in the avelumab arm and 19.2 month median follow-up in the best supportive care arm. At this time, 24% of 350 patients in the avelumab arm were still receiving therapy, and 7% of 350 patients in the best supportive care arm were “on treatment”. The median duration of treatment with avelumab was 24.9 weeks, and median duration in best supportive care arm was 13.1 weeks. Disease progression was the most likely cause for discontinuation.
The overall survival data are shown below.
The study met its overall endpoint of overall survival benefit with avelumab maintenance therapy, with a median overall survival of 21.4 months versus 14.3 months in the supportive care arm. The hazard ratio for death with avelumab was 0.69. At 12-months, 71% of patients in the avelumab maintenance arm were alive relative to 58% of patients in the supportive care arm. A similar result was observed for survival in the PD-L1+ population.
Subgroup analyses, shown below, generally favored avelumab.
Independent radiology review confirmed a radiographic progression-free survival advantage for the avelumab treatment arm in the overall population as well as PD-L1+ group, with a subset of patients displaying a progression plateau.

Importantly, for patients who discontinued therapy due to progressive disease, only 52.9% of patients in the best supportive care arm went on to receive a PD-L1 or PD-1 inhibitor, which would be standard of care.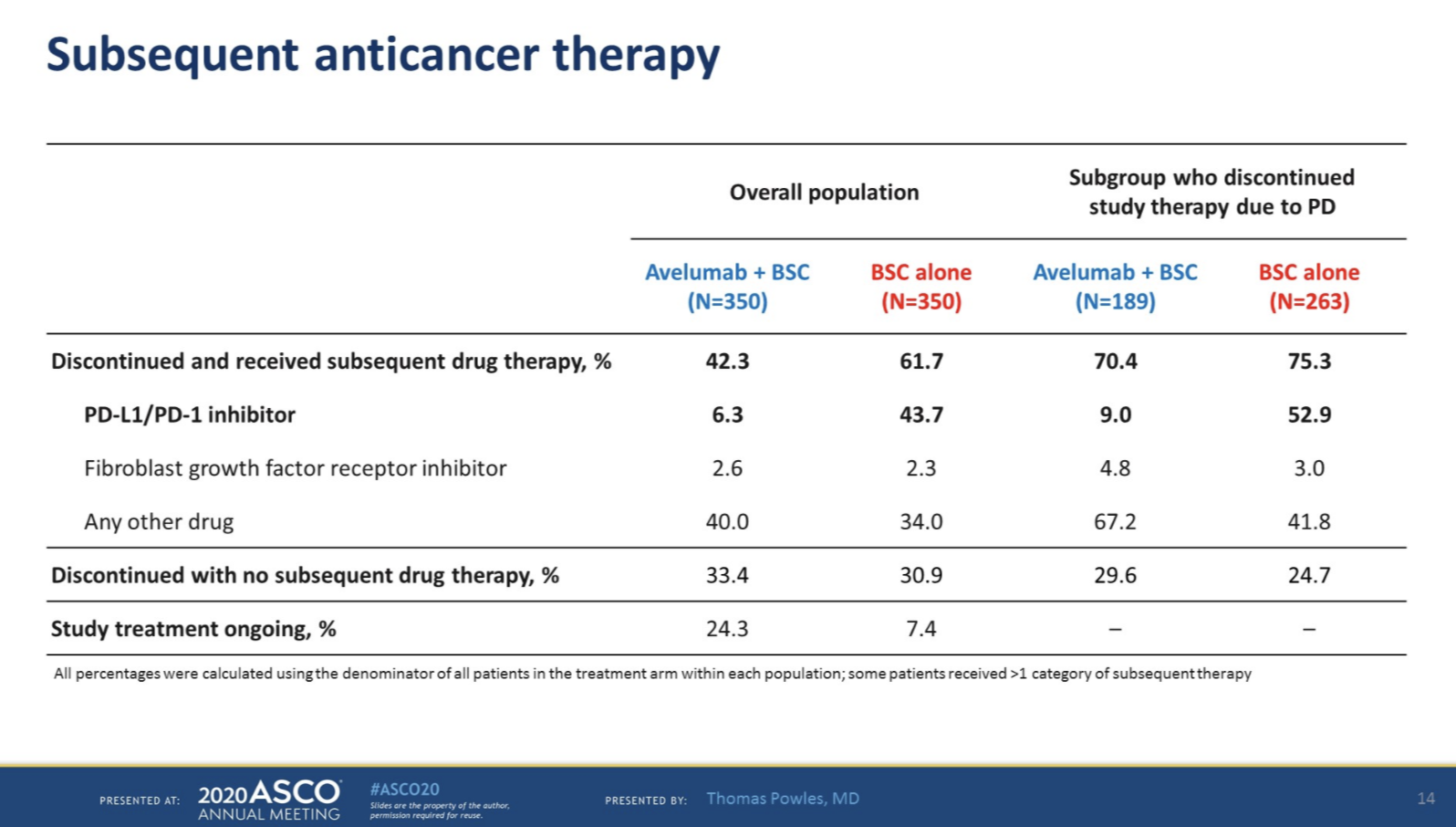
Treatment-emergent adverse events are shown below. Overall more Grade 3+ adverse events were noted in the avelumab treatment arm.
Hypothyroidism was the most common immune-related adverse event. 9% of patients required corticosteroid treatment for their immune-related adverse event.
In summary, the JAVELIN Bladder 100 trial demonstrated significantly longer overall patient survival with first-line maintenance avelumab versus best supportive care in patients with metastatic urothelial cancer who achieved at least stable disease with at least 4 lines of first-line platinum-based chemotherapy. This survival benefit was observed with both cisplatin and carboplatin initial therapy, and in patients who had stable disease, partial response, or complete response to chemotherapy. No new safety signals were observed with avelumab maintenance therapy.
Dr. Powles stated that based on these findings, avelumab as first-line maintenance therapy is a new standard of care for patients with advanced urothelial cancers who have not progressed on platinum-based induction therapy.
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London, United Kingdom
Written by: Alok Tewari, MD, Ph.D., Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29- 31, 2020
Related Content:
Watch: JAVELIN Bladder 100: Avelumab for Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma - Thomas Powles
Read: Phase III Trial Shows Avelumab an Immunotherapy Treatment for Advanced Urothelial Cancer Prolongs Overall Survival
Read: BAVENCIO® Receives FDA Breakthrough Therapy Designation for First-Line Maintenance Treatment of Locally Advanced or Metastatic Urothelial Carcinoma.
Read: ASCO 2020: Checkpoint Inhibition in Metastatic Urothelial Carcinoma: Timing is Everything
JAVELIN Bladder 100 Abstract Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 18.


