(UroToday.com) There are currently no FDA approved neoadjuvant therapies for patients with locally advanced renal cell carcinoma (RCC). Patients with high-risk disease, such as those with T3 or N1 disease have 5-year survival rates of 61% and some suggest that lymph node-positive stage III disease have similar survival to patients with stage IV disease1. Immune checkpoint inhibitors have demonstrated overall survival in the metastatic setting in both the first line and second-line setting. This study evaluates the safety and efficacy of neoadjuvant immunotherapy for patients with locally advanced RCC.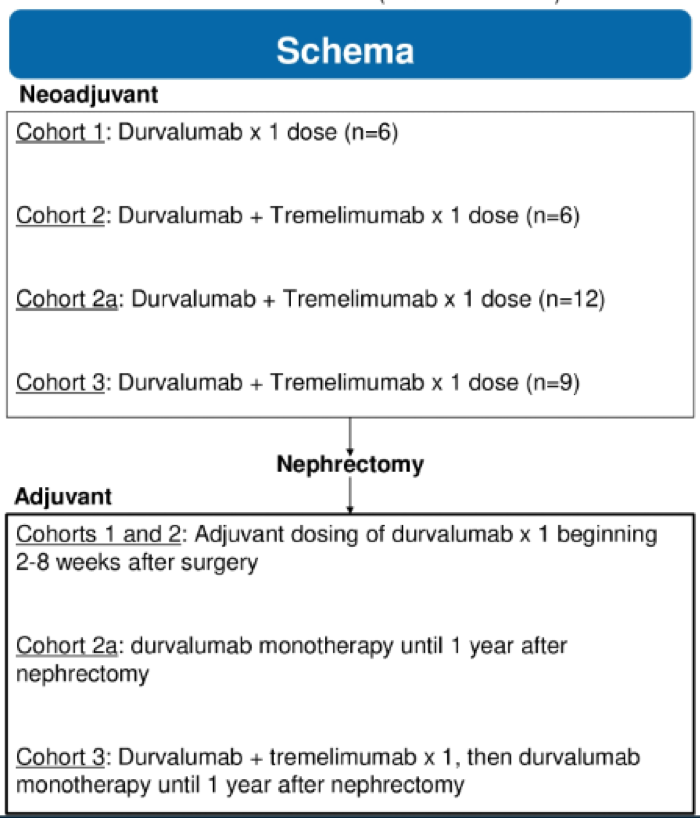
The treatment schema is shown above. Patients were enrolled in four separate cohorts in the neoadjuvant setting, followed by nephrectomy, and then received three forms of adjuvant immunotherapy after nephrectomy. The key inclusion criteria included patients with T2B-4 and/or N1, MO disease. The primary objective of this study was the safety and feasibility of neoadjuvant and adjuvant dosing of durvalumab and tremelimumab and secondary objectives were to assess the immune response measured by the density of tumor-infiltrating CD8 T cells and change in tumor size.

Baseline characteristics are shown above. 28% had lymph node-positive disease and 93% were T3 or higher. 13% of patients had a sarcomatoid component. In terms of pathological staging, 85% of patients had a T3 or higher disease after neoadjuvant therapy and 15% continued to have lymph node disease.

In terms of safety, there was a high rate of treatment discontinuation due to adverse events, especially in cohorts 2A and 3, with 7/17 patients requiring treatment with high dose steroids. Due to the high number of treatment discontinuation, the study was suspended.

In terms of the immune related side effects, again, cohorts 2A (D+T -> surgery -> D x 1 year) and 3 (D+T, -> surgery -> D+T -> D x 1gear) had the most AEs, most common being hyper/hypothyroidism, elevated lipase/amylase, rash, and elevated AST/ALT.
Single-agent durvalumab was better tolerated than combination durvalumab/ tremelimumab, as expected, for patients with locally advanced RCC. Still, even with single agent, 1/6 patient had to discontinue due to adverse events. There did not appear to be much downstaging with neoadjuvant immunotherapy but with only one cycle I don’t believe that was to be expected, especially since median time from neoadjuvant therapy to nephrectomy was only 1 week. Importantly, there were no treatment-related delays to nephrectomy or surgical complications. Correlative biomarker analysis is pending.
Presented by: Moshe Chaim Ornstein, MD, MA, Genitourinary Oncologist, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Written by: Jason Zhu, MD. Medical Oncologist, Division of Genitourinary Cancers, Levine Cancer Institute, Twitter: @TheRealJasonZhu, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
References:
- Srivastava A, Rivera‐Núñez Z, Kim S, et al. Impact of pathologic lymph node–positive renal cell carcinoma on survival in patients without metastasis: Evidence in support of expanding the definition of stage IV kidney cancer. Cancer 2020.


