
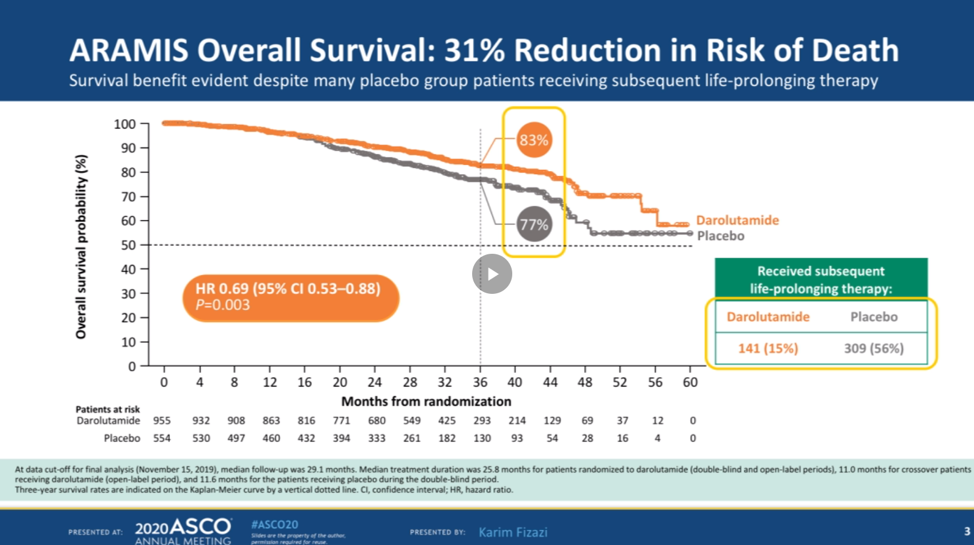
Data on 1509 patients is presented. The overall survival analysis was conducted after 254 deaths were observed in the study. Cross-over was allowed in the study and 170 patients receiving placebo crossed over to darolutamide. In terms of overall survival, darolutamide had a 31% reduction in the risk of death compared with placebo alone. At 3 years, 93% of patients were alive in the darolutamide arm compared with 77% of patients in the placebo arm. The benefit in overall survival is seen despite half the patients in the placebo arm subsequently receiving darolutamide or active therapies.
Darolutamide also significantly improved time to pain progression (40.3 months vs 25.4 months, HR 0.65, p <0.001), time to first cytotoxic chemotherapy (HR 0.58), and time to first symptomatic skeletal event (0.48).

No new safety signals emerged with extended follow up and the safety profile was consistent with the initial analysis. In terms of fatigue, only 0.4% of patients had Grade 3/4 fatigue with darolutamide, compared with 0.9% of patients on placebo. One of the major advantages of darolutamide over other androgen receptor inhibitors is that darolutamide does not cross the blood-brain barrier. This resulted in no differences in falls or CNS events compared with placebo. Patients with seizures were allowed to enroll in this study, and there was no difference in the incidence of seizure between either arm. There were greater cardiac arrhythmias in the darolutamide arm than placebo – however, the baseline characteristics of the patients revealed greater cardiac comorbidities in the darolutamide group than the placebo group which may help explain this difference.

Extended follow up of ARAMIS demonstrates that the addition of darolutamide to ADT not only improves metastasis-free survival, but also improves overall survival, time to pain progression, time to chemotherapy, and time to symptomatic skeletal events. Darolutamide may be better tolerated than other drugs in its class due to its decreased penetration of the blood-brain barrier and safety analysis shows no major differences in seizures, dizziness, or cognitive impairment between darolutamide and placebo. Given these results, darolutamide is a great option for all patients with nmCRPC and should be considered the drug of choice for patients with underlying neurological comorbidities or fatigue at baseline.

Presented by: Karim Fizazi, MD, Ph.D., Head of the Department of Cancer Medicine at the Institut Gustave Roussy, Villejuif, France and Professor of Oncology at the University of Paris
Written by: Jason Zhu, MD. Medical Oncologist, Division of Genitourinary Cancers, Levine Cancer Institute Twitter: @TheRealJasonZhu at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29- 31, 2020
References:
Related Content:
View: Darolutamide Treated Patients Maintained Quality of Life in ARAMIS Trial - Fred Saad
View: ARAMIS - Efficacy and Safety of Darolutamide in nmCRPC - Karim Fizazi
View: ARAMIS Trial: Darolutamide Demonstrates Improved Overall Survival in Nonmetastatic Castration-Resistant Prostate Cancer (nmCRPC) - Fred Saad


