In the REASSURE study, patients were enrolled only if they had histologically or cytologically confirmed metastatic castrate-resistant prostate cancer and were scheduled to receive radium 223 by their treating physician. Patients previously treated with radium 223 or who were scheduled to receive other radiopharmaceuticals were excluded from the study.
A total of 1465 patients from all over the world were included in the REASSURE safety analysis. 12% (182 patients) of the patients received taxane chemotherapy subsequent to radium 223 therapy, with 73 patients (40%) receiving chemotherapy immediately after and 109 patients (60%) receiving it with a delay after radium 223 completion. The median time from the last dose of radium 223 until beginning chemotherapy was 42.5 (8-90) days for the immediate group and 222 (91-863) days for the delayed group. Patient demographic data are shown in Table one.
Table 1 - Demographic data:
When assessing treatment sequencing, most patients (99%) received prior systemic anti-cancer therapy (Figure 2). 90% of the patients received concomitant systemic anti-cancer therapy in addition to radium 223, which included abiraterone and enzalutamide. Subsequent systemic anti-cancer therapy after completion of radium 223 included enzalutamide, abiraterone, and chemotherapy (cabazitaxel, and docetaxel) as shown in Figure 3.
Figure 2 – Prior therapies: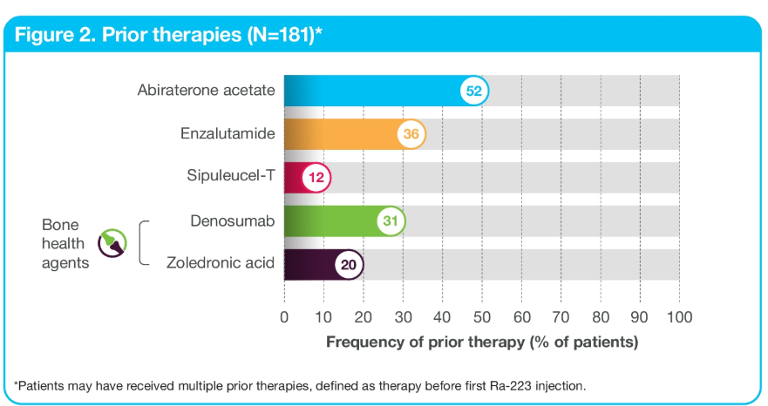
Figure 3 – Subsequent therapies: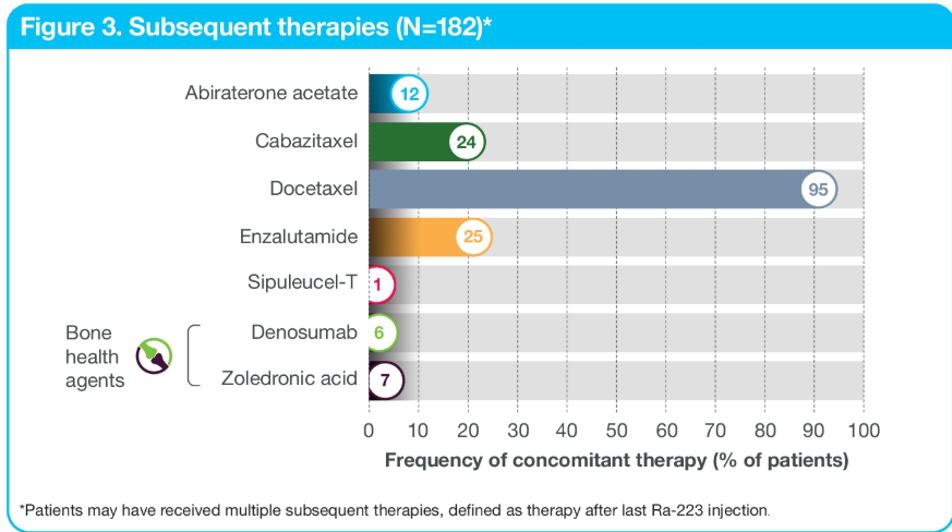
When assessing the adverse events in the 182 patients who received subsequent taxane chemotherapy, the incidence of drug-related adverse events and serious adverse events during treatment with the radium 223 was similar between the immediate and delayed chemotherapy groups, as shown in Figure 4A. In the 182 patients who received subsequent taxane chemotherapy 8%, 5% and 9% of patients in the immediate and delayed groups, respectively, reported grade three or four haematologic adverse events during subsequent chemotherapy, as shown in Figure 4B.
The median overall survival was 24.3 months from initiation of radium 223 treatment and 11.8 months from the start of subsequent chemotherapy, as shown in Figure 5.
The authors concluded their presentation by summarizing the data that showed that many patients received multiple subsequent anti-cancer therapies, including abiraterone in 12% of cases and enzalutamide in 25% of cases. The median overall survival was more than two years from the start of treatment with radium 223 and almost one year from the start of chemotherapy. The study shows that taxane chemotherapy in routine clinical practice given to patients previously treated with radium 223 has an acceptable hematological safety profile, regardless of whether chemotherapy was received immediately or in a delayed manner, following radium 223 therapy. Therefore, the authors conclude that radium 223 is a viable treatment option in patients with bone metastasis with chemotherapy remaining an option given the observed acceptable safety profile.
Figure 4 – Incidence of adverse effects

Figure 5 – Overall survival:
Presented by: Celestia S. Higano, MD, FACP, Department of Medicine, University of Washington and Fred Hutchinson Cancer Research Center, Seattle, WA
Written By: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29- 31, 2020
References:
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet (London, England) 2010; 376(9747): 1147-54.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. New England Journal of Medicine 2004; 351(15): 1502-12.
- Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. New England Journal of Medicine 2013; 369(3): 213-23.


