(UroToday.com) Cell-free DNA (cfDNA) levels were previously shown to correlate with radiographic progression-free survival (rPFS) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving taxanes in the Phase III FIRSTANA1 and PROSELICA2 trials. ctDNA is a constituent of cfDNA released into the blood of tumor cells and can provide insight into the genomic heterogeneity of cancerous lesions. Furthermore, ctDNA levels vary during treatment and may correlate with disease response and relapse. At the 2020 American Society of Clinical Oncology (ASCO) virtual meeting, Dr. Goodall and colleagues presented results of their evaluation of ctDNA in serial plasma samples from patients enrolled in A.MARTIN, a randomized Phase II study of abiraterone with or without ipatasertib in patients with mCRPC.
The A.MARTIN Phase II trial schema is as follows: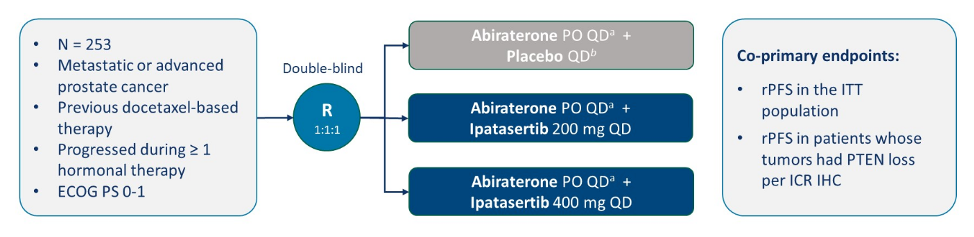
In this trial, Dr. Johann de Bono and colleagues previously showed that combined ipatasertib and abiraterone improved radiographic progression-free survival in patients with mCRPC with PTEN loss.3 For this analysis, blood was collected in cell-free DNA Streck tubes from 216 patients at three time points; baseline, C3D1, and end of treatment. The liquid biopsy analysis in the A.MARTIN trial is summarized as follows:
cfDNA was extracted from plasma using a Circulating DNA Kit (Qiagen) on a QIASymphony machine (Qiagen). Twenty-five nanograms of extracted cfDNA was used in library preparation, constructed with a custom-designed, 58 gene, QIAseq Targeted DNA panel enriched for PI3K/AR pathway genes. Samples were sequenced to mean depth of 3394x on a NextSeq 500 machine.
Baseline ctDNA analysis detected a number of somatic mutations including TP53, AR, FOXA1, PTEN, and SPOP as the most frequently mutated genes. Additionally, baseline ctDNA positivity correlated with rPFS: hazard ratio [HR] 1.9, 95% confidence interval [CI] 1.3-2.7. This association with rPFS was maintained in a multivariate cox model with more than five baseline clinical variables (HR 1.9, 95% CI 1.2-2.9). Patients with a C3D1 reduction in ctDNA had superior rPFS compared to patients with a C3D1 increase in ctDNA (HR 2, 95% CI 1.3-3.2). The rate of ctDNA clearance at C3D1 was higher in the ipatasertib 400mg arm compared to placebo (56.3% versus 24.4%, p < 0.01). Furthermore, changes in ctDNA were associated with best confirmed overall response: complete response/partial response patients had the greatest reduction in ctDNA (mean of -18.1%), followed by stable disease and/or prostate-specific antigen (PSA) response (-15.9%), stable disease and/or no PSA response (-7.0%), and lastly progressive disease patients (0.8%). Patients who remained ctDNA negative had the best rPFS, followed by patients experiencing a reduction in ctDNA; higher post-treatment ctDNA significantly correlated with worse rPFS (ctDNA increase vs always ctDNA negative: HR 2.89, 95% CI 1.74-4.78):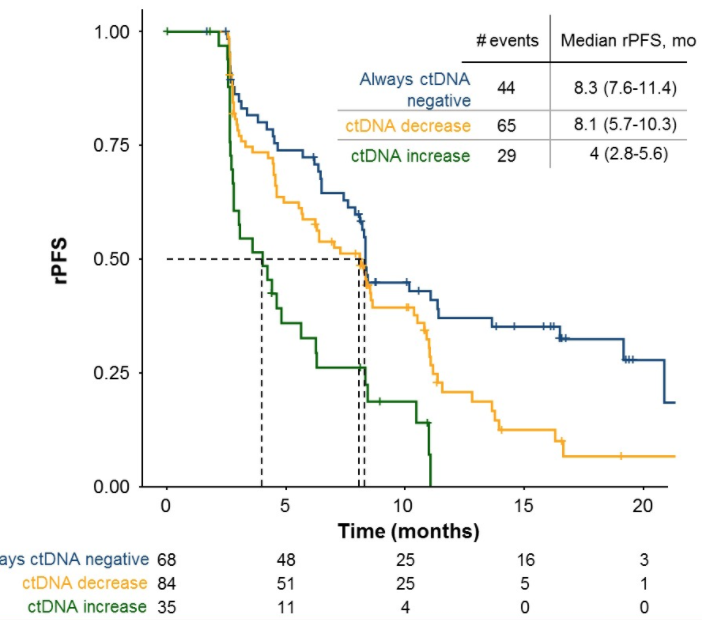
Dr. Goodall concludes this presentation with the following concluding statements:
- Baseline ctDNA positivity was associated with worse rPFS
- Reductions in ctDNA with treatment were associated with improved rPFS
- Serial ctDNA analyses informed on disease response and progression
- Further validation is required and is ongoing with the Phase III IPATential150 study evaluating ipatasertib plus prednisone vs placebo plus abiraterone and prednisone in patients with mCRPC
Co-Authors: Zoe June Assaf, Zhen Shi, George Seed, Liangxuan Zhang, Ben Lauffer, Wei Yuan, Matthew Wongchenko, Flavia Oliveira, Suzanne Carreira, Steven Gendreau, Johann S. De Bono; The Institute of Cancer Research, London, United Kingdom; Genentech, South San Francisco, CA; Genentech, Inc., South San Francisco, CA; Institute of Cancer Research, London, United Kingdom; Genentech, Inc., South San Francisico, CA; The Royal Marsden Hospital and The Institute of Cancer Research, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md, at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020
References:
1. Oudard, Stéphane, Karim Fizazi, Lisa Sengeløv, Gedske Daugaard, Fred Saad, Steinbjørn Hansen, Marie Hjälm-Eriksson et al. "Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA." Journal of Clinical Oncology 35, no. 28 (2017): 3189-3197.
2. Eisenberger, Mario, Anne-Claire Hardy-Bessard, Choung Soo Kim, Lajos Géczi, Daniel Ford, Loïc Mourey, Joan Carles et al. "Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA." Journal of Clinical Oncology 35, no. 28 (2017): 3198-3206.
3. de Bono, Johann S., Ugo De Giorgi, Daniel Nava Rodrigues, Christophe Massard, Sergio Bracarda, Albert Font, Jose Angel Arranz Arija et al. "Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss." Clinical Cancer Research 25, no. 3 (2019): 928-936.


