Radium 223, which is an alpha particle emitter, is an approved treatment option for metastatic castrate-resistant prostate cancer patients with symptomatic bone metastasis, as previously demonstrated in the ALSYMPCA trial.2
Niraparib is a potent and selective PARP 1/2 inhibitor with a favorable safety profile and has shown single-agent activity in castrate-resistant prostate cancer. However, data on the safety and efficacy of this type of treatment combined with radiotherapy is still lacking.
It has been suggested that PARP inhibition may further enhance the clinical benefits caused by radium 223. In this study, the authors hypothesized that targeting the PARP/Androgen receptor access in combination with radium 223 is a tolerable treatment option and will eventually result in improved management of metastatic castrate-resistant prostate cancer patients, independent of their DNA damage repair status.
In this specific phase 1B study (NCT03076203, the study design is shown in Figure 1), the primary objective of the authors was to determine the optimum dose of niraparib when combined with radium 223 in metastatic cancer-resistant prostate cancer patients, regardless if they had received previous chemotherapy. The secondary objectives included determining the proportion of patients with 50% or more PSA reduction after 12 weeks, overall progression-free survival, radiographic progression-free survival, proportion of patience that had circulating tumor cell (CTC) conversion, time to total alkaline phosphatase progression, total alkaline phosphatase normalization and long-term safety and tolerability of niraparib + radium 223.
Figure 1:
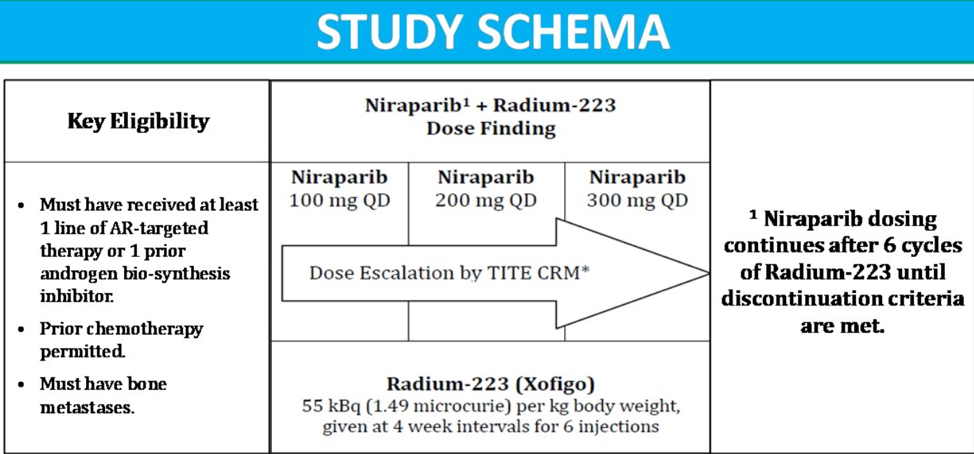
This was a phase 1B dose-finding study consisting of patients with progressive metastatic castrate-resistant prostate cancer, which attempted to identify the maximum tolerated dose of niraparib based on toxicities observed over 12 weeks of treatment. Patients were enrolled in one of three dose levels of niraparib (100, 200, and 300 mg PO daily, as shown in Table 1). Upon completing six cycles (with 4-week intervals) of radium 223, patients continued with niraparib treatment alone until objective progression, treatment intolerance, or patient decision to stop treatment.
Table 1 – Treatment options:
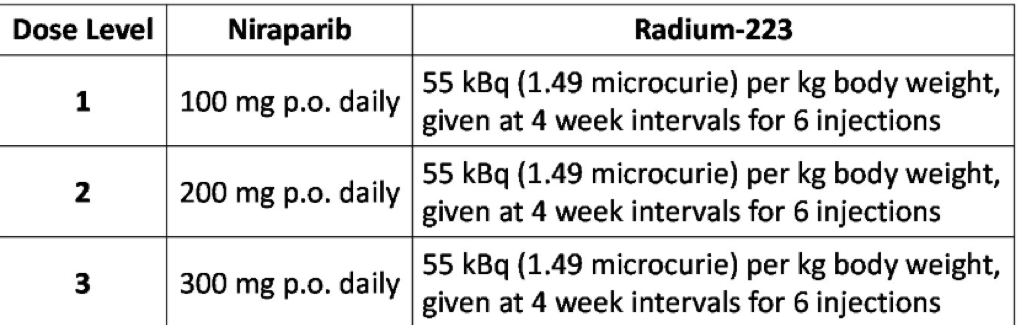
A total of 30 patients had been enrolled between October 2017 and January 2020. 15 patients were in the chemotherapy-naive arm and 15 patients in the chemotherapy exposed arm. The median age was 70 years (range 50-83), and the median ECOG performance status was 0 (range 0-1). The maximum tolerated those of niraparib was 100 milligrams in the chemotherapy exposed arm in 200 milligrams in the chemotherapy-naive arm.
Table two demonstrates the median time on treatment and the proportion of patients who achieved over 50% PSA decline at 12 weeks and over 30% alkaline phosphate decline for each cohort and arm.
Table 2 – Median time on treatment and proportion of patients with over 50% PSA decline at 12 weeks, and over 30% decline in alkaline phosphatase overall for each arm and cohort:
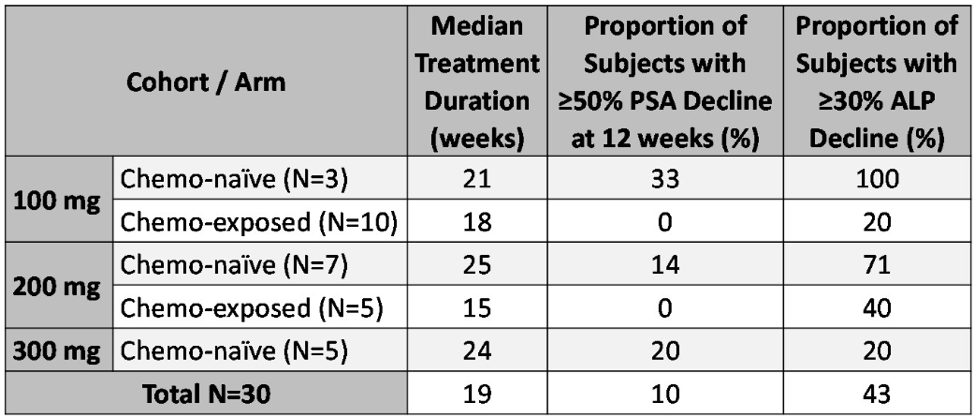
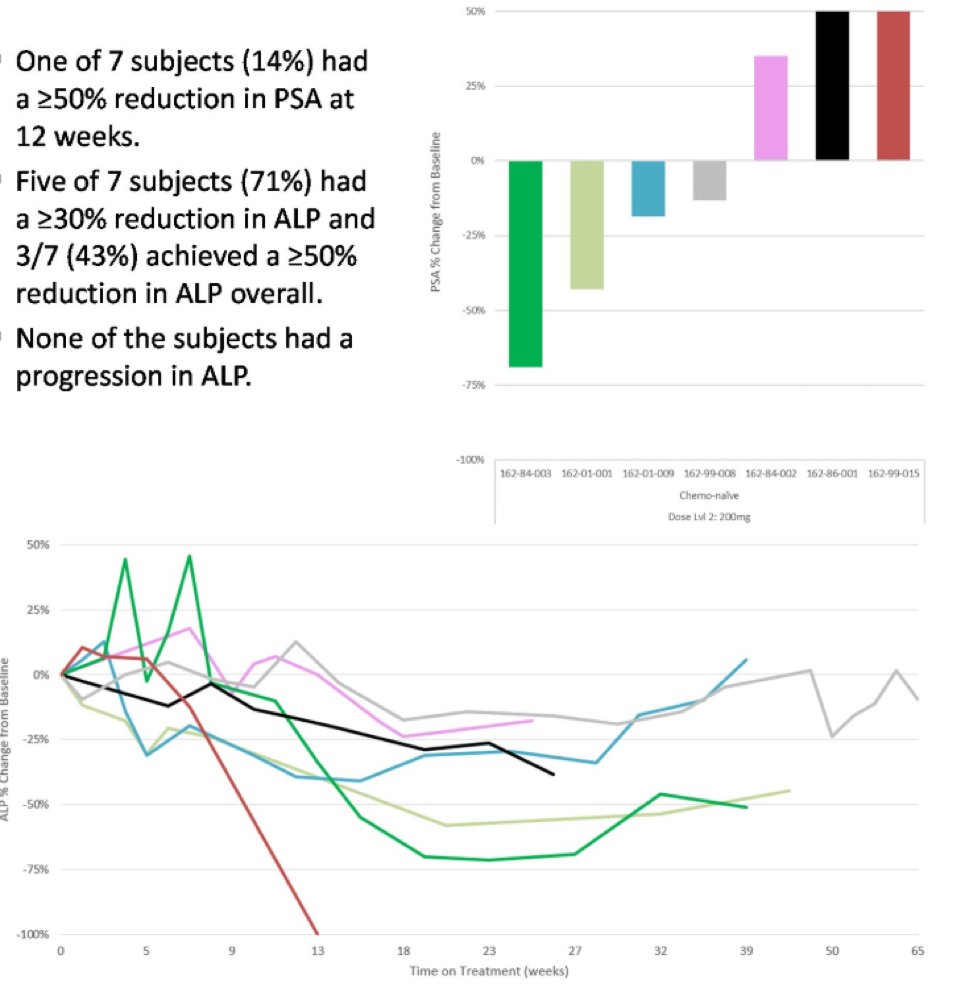
Figure 2 demonstrates the PSA percent change from baseline to 12 weeks and the change in total alkaline phosphate overtime for the chemotherapy-naive patients, being treated with the maximum tolerated dose of niraparib (200 milligrams daily).
Figure 2:
The authors concluded by stating that the combined treatment with niraparib and radium 223 was shown to be safe and tolerable. The maximum tolerated dose of niraparib was identified, indicating that further investigation of the 200-milligram dose in the chemotherapy-naive setting is warranted in a phase two study.
Presented by: William Kevin Kelly, Sidney Kimmel Cancer Center at Thomas Jefferson University, Philadelphia, PA
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan
References:
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine 2020; 382(22): 2091-102.
- Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. New England Journal of Medicine 2013; 369(3): 213-23


