(UroToday.com) The mutational landscape of metastatic castration-resistant prostate cancer (mCRPC) is generally quiescent compared to other solid tumors. DNA methylation, an epigenetic mark on cytosine residues in CpG dinucleotides, can change the activity of a DNA segment without changing the DNA sequence. When located in a gene promoter, DNA methylation typically acts to repress gene transcription. Furthermore, DNA methylation results from the balance of two classes of enzymes: the DNMT enzymes that control de novo methylation and the TET enzymes that control active demethylation. The West Coast Dream Team is supported by the Prostate Cancer Foundation and Stand Up 2 Cancer, which has collected more than 400 annotated metastatic biopsy samples from mCRPC patients. At the 2020 virtual ASCO annual meeting, Eric Small, MD, and colleagues presented results defining the comprehensive methylation landscape of mCRPC.
For this study, mCRPC patients underwent a metastasis biopsy as part of a multi-institutional study. Among 387 first biopsies, 302 were positive, and 174 were selected for LCM and RNA-seq. Subsequently, deep whole-genome bisulfite sequencing was performed on fresh frozen tissue from 100 mCRPC patients. As follows is the site of biopsies to date from the total cohort:
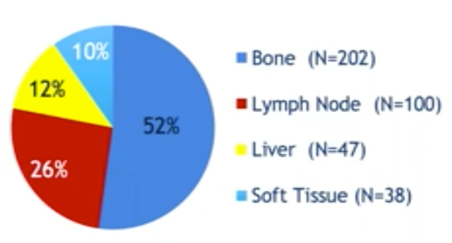
Data was then paired with deep whole-genome and transcriptome sequencing from the same samples. Unbiased hierarchical clustering of the mCRPC methylome was undertaken, and the survival of patients in each cluster was calculated using the Kaplan Meier method.
Unbiased hierarchical clustering revealed several distinct subtypes. Benign prostate tissue had the least hypomethylation, primary prostate cancer had intermediate hypomethylation, and mCRPC patients had the highest hypomethylation. There were 22% of mCRPC samples that exhibited a novel epigenomic subtype associated with hyper-methylation (CMP subtype). This hypermethylated cluster was significantly associated with somatic mutations in genes known to be involved in methylation, for example, TET2, and DNMT3B, as well as in genes in which mutations have been associated with hyper-methylation in other cancer types (IDH1 in glioblastoma and BRAF in colon cancer). mCRPC survival was 56.2 months in patients with hypermethylated cancers (cluster 3 + CMP) compared to 35.6 months in non-hypermethylated (cluster 1 + cluster 2) (p = 0.047). Furthermore, on multivariable analysis, hypermethylated cancers (cluster 3 + CMP) was protective for mortality (HR 0.514, p=0.043). Methylome clustering also identified a unique cluster comprised of all patients with treatment-induced small cell/neuroendocrine cancer, a subtype previously associated with poor survival.
Dr. Small concluded his presentation with the following take-home messages:
- This integrated study of the whole-genome, whole methylome and whole-transcriptome sequencing provides the first comprehensive overview of the important regulatory role of methylation in metastatic castration-resistant prostate cancer
- CMP comprised 22% of the samples, which is characterized by somatic mutations in IDH1, BRAF, TET2, and DNMT3B
- A larger hypermethylated cohort, comprising CMP patients plus patients in cluster 3 had superior survival to patients that were not hypermethylated
- The improved survival observed with larger hypermethylated cohorts was an independent predictor of OS in multivariable analysis
- mCRPC can be stratified by methylation pattern in three mutually exclusive prognostic groups: hypermethylated adenocarcinoma, non-hypermethylated adenocarcinoma, and treatment associated small cell/neuroendocrine cancer
Presented by: Eric J. Small, MD, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Co-Authors: Shuang Zhao, William S. Chen, Haolong Li, Adam Foye, Martin Sjöström, Jun Jie Hua, Rahul Raj Aggarwal, Joshi J. Alumkal, Tomasz M. Beer, Martin Gleave, Matthew Rettig, Owen Witte, Primo Lara, Arul Chinnaiyan, Chris Maher, David A. Quigley, Felix Y Feng; UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA; Univerisity of Michigan, Baltimore, MI, Cayman Islands; UC San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA; UCSF, San Francisco, CA; Lund University, Department of Oncology and Pathology, Lund, Sweden; OHSU Knight Cancer Institute, Portland, OR; Knight Cancer Institute, Oregon Health & Science University, Portland, OR; Vancouver Prostate Centre, University of British Columbia, Vancouver, BC, Canada; UCLA's Jonsson Comprehensive Cancer Center, West Los Angeles VA Medical Center, Los Angeles, CA; UCLA, Los Angeles, CA; University of California, Sacramento, CA; University of Michigan Medical School, Ann Arbor, MI; Washington University School of Medicine in St. Louis, St. Louis, MO; Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.


