To date, most guidelines have recommended the use of conventional imaging approaches using bone scintigraphy (bone scan) and computed tomography or magnetic resonance imaging for the detection of bony lesions and soft tissue (nodal or visceral) lesions, respectively. However, these approaches are limited by both poor sensitivity and relatively poor specificity.
The use of positron emission tomography (PET) scanning, particularly using a prostate-specific membrane antigen (PSMA), has increased dramatically in the past few years. There are a variety of different tracers used with varying test characteristics. In each case, these tend to outperform conventional imaging.
In the Genitourinary Cancer—Prostate, Testicular, and Penile Oral Abstract Session at the 2020 American Society of Clinical Oncology Virtual Annual Meeting, Dr. Michael Morris and colleagues presented the results of their CONDOR trial, a study of 18F-DCFPyL PET/CT imaging in patients with suspected recurrence of prostate cancer. This is a multi-center, open-label, single-arm Phase III trial aimed at identifying improved imaging approaches for men with biochemical recurrence following local therapy for prostate cancer and fits as part of a larger clinical development program.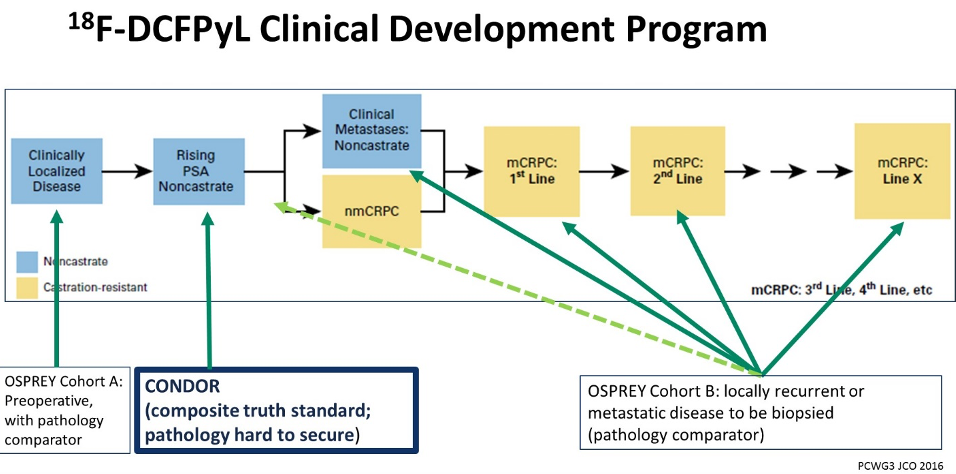
Across several institutions, the authors recruited men aged 18 years and older with rising prostate-specific antigen (PSA) after definitive therapy and negative or equivocal standard of care imaging (e.g., CT/MRI, bone scintigraphy).
The authors undertook by PyL-PET/CT using a single 9 mCi (333 MBq) ± 20% dose of PyL, followed by PET/CT one to two hours later.
As their primary outcome of interest, the authors assessed the correct localization rate (CLR), defined as the percentage of patients with a 1:1 correspondence between at least one lesion identified by PyL-PET/CT and the composite standard of truth: pathology, correlative imaging, or PSA response. Secondarily, the authors assessed the effect of PyL-PET/CT on the clinical management by determining changes in the treating physician’s documented clinical plans before and after PyL-PET/CT.
In this single-arm study, the authors specified that the trial would be considered successful if the lower bound of the 95% confidence interval for CLR exceeded 20% for two of three independent, blinded central PyL-PET/CT reviewers.
The authors accrued 208 men who met inclusion criteria. The median PSA in this cohort was 0.8 [0.2 - 98.4] ng/mL. Using their defined primary outcome of correct localization rate, the authors demonstrated that PyL-PET/CT correctly localized lesions in 84.8-87.0% of cases among the three readers. The lower limit of the 95% confidence interval of the CLR exceeded 77% for all three readers, thus meeting the pre-specified outcome criteria.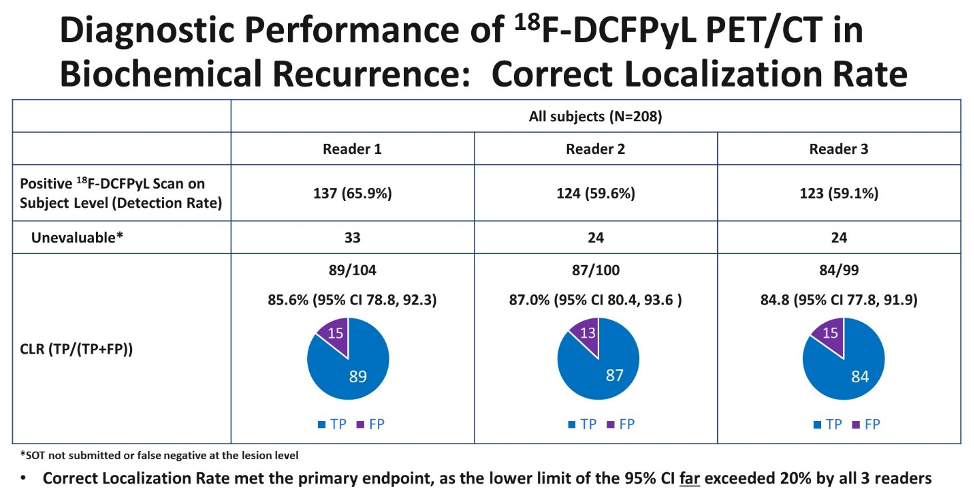
While these imaging characteristics are important, potentially more important is the demonstrated clinical effect of this information. Using local radiographic assessment, PSMA-avid lesion(s) were found in 142 of 208 patients (69.3%). As a result, more than two-thirds of patients enrolled in this study (131 of 205, 63.9%) had a change in intended management following PyL-PET/CT. Of those with a change in management, nearly 80% (103/131, 78.6%) were attributable to positive PyL findings while the remaining 21.4% (28/131) were attributable to negative PyL scans. Changes in management included a transition from salvage local therapy to systemic therapy based on more extensive disease (n=58), a period of observation (n=49), non-curative systemic therapy to attempted curative salvage local therapy (n=43) and observation in place of planned treatment (n=9).
In addition to providing this information, PyL was well tolerated with one drug-related serious adverse event (SAE) (hypersensitivity) and the most common adverse event (AE) being headache (n=4; 1.9%).
As a result, the authors conclude that PSMA-targeted PyL-PET/CT detected local and metastatic recurrence in the majority of men with BCR presenting with negative or equivocal conventional imaging. As a result of the imaging results, most men had changes in recommended treatment approaches.
Presented by: Michael J. Morris, MD, Clinical Director, Genitourinary Medical Oncology Service & Prostate Cancer Section Head, Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, New York
Co-Authors: Peter R. Carroll, Lawrence Saperstein, Frederic Pouliot, David Josephson, Jeffrey Y.C. Wong, Austin R. Pantel, Steve Y. Cho, Kenneth Gage, Morand Piert, Andrei Iagaru, Janet H. Pollard, Vivien Wong, Jessica Donato Jensen, Nancy Stambler, Michael A. Gorin, Barry Siegel
Written by: Christopher J.D. Wallis, MD, Ph.D., Urologic Oncology Fellow, Vanderbilt University Medical Center, Nashville, Tennessee, Twitter: @WallisCJD, at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020


