For this study, patient-level data from the ARAMIS (darolutamide versus placebo, n=1,509), SPARTAN (apalutamide versus placebo, n=1,207) and PROSPER (enzalutamide versus placebo, n=1,401) trials were used.
Safety outcomes assessed included:
- Any grade adverse events
- Any serious adverse events
- Adverse events leading to treatment discontinuation
- Adverse events leading to/associated with death
- Individual adverse events of special interest in nmCRPC selected by clinical experts or considered to have CNS relevance
Individual patient-level data from ARAMIS were selected and re-weighted to match the inclusion criteria and baseline characteristics published in SPARTAN and PROSPER since the authors did not have access to these trial’s individual patient-level data. Furthermore, the Benjamini-Hochberg approach was applied to adjust for multiplicity. The darolutamide versus apalutamide matching-adjusted indirect comparison matched on seven covariates: age, PSA level, and doubling time, Eastern Cooperative Oncology Group (ECOG) score, Gleason score, bone-sparing agent use, and prior surgery. Sensitivity analyses were conducted matching on different sets of covariates. Darolutamide versus enzalutamide were matched on age, region, PSA level and doubling time, ECOG score, Gleason score, and bone-sparing agent use. Risk difference ([darolutamide – placeboARAMIS] – [enzalutamide – placeboPROSPER]) and odds ratio (ORARAMIS/ORPROSPER) were calculated. Risk difference <0 or odds ratio <1 indicated lower adverse event risk for darolutamide.
For the general safety outcomes, no statistically significant differences were observed between darolutamide and apalutamide after matching. However, darolutamide showed a numeric advantage over apalutamide on adverse events leading to treatment discontinuation and adverse events leading to death, whereas apalutamide showed a numeric advantage over darolutamide on any serious adverse events. Among individual adverse events, darolutamide generally exhibited favorable results compared to apalutamide, showing advantages for mental-impairment disorder, diarrhea, nausea, fatigue, and severe fatigue, as well as reaching statistical significance for falls, rash and fracture: 

For the general safety outcomes, there were no statistically significant differences observed between darolutamide and enzalutamide after matching, although darolutamide showed a numeric advantage over enzalutamide on any serious adverse events, adverse events leading to treatment discontinuation, and adverse events leading to death. Among individual adverse events, darolutamide generally exhibited favorable results compared to enzalutamide, showing an advantage for nausea, asthenia, and headache, as well as reaching statistical significance for falls, dizziness, mental-impairment disorder, hypertension, fatigue (all grades), and severe fatigue: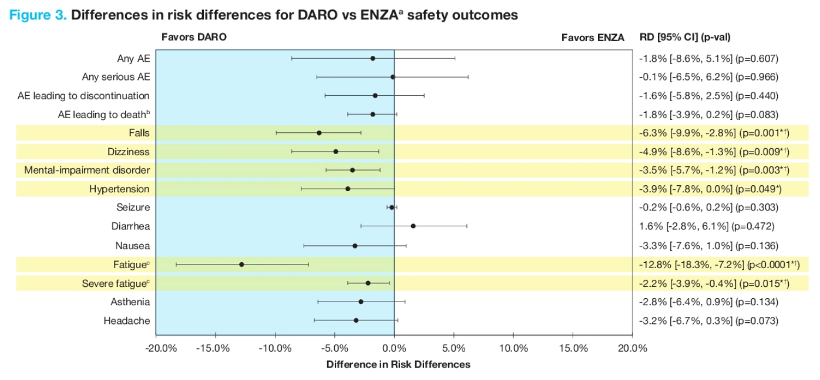

The strengths of this study include matching-adjusted indirect comparisons being utilized to adjust for observed cross-trial differences in ARAMIS, SPARTAN, and PROSPER trials. Furthermore, using ARAMIS patient-level data-enabled granularity in data handling for greater comparability with SPARTAN and PROSPER. Several limitations include (i) only known baseline factors that were consistently reported across trials were included among the matching covariates in the matching-adjusted indirect comparisons, (ii) as with any comparison of non-randomized treatment groups, such comparisons are subject to potential bias due to unobserved or unmeasurable confounding factors, and (iii) the results of the study may not be generalizable beyond the study sample.
Conclusions from this analysis of matching-adjusted indirect comparison for darolutamide versus apalutamide and enzalutamide are as follows:
- After adjusting for trial differences and multiple testing, darolutamide showed a generally favorable safety profile when compared to apalutamide and enzalutamide
- Darolutamide had a statistically significant lower risk of falls, fracture, and rash compared to apalutamide
- Darolutamide had a lower risk of falls, dizziness, mental impairment, fatigue, and severe fatigue compared to enzalutamide
- These results are consistent with darolutamide’s reduced risk of crossing the blood-brain barrier
- While head-to-head trials are the gold standard in comparative clinical evaluations, these results are useful in informing shared decision-making between the patient and clinician
Presented by: Shan Jiang, PhD, Bayer U.S. LLC, Whippany, NJ
Co-Authors: Emi Terasawa, Viviana Garcia Horton, Rajeev Ayyagari, A. Reginald Waldeck, Susan Halabi, Neal D. Shore; Bayer U.S. LLC, Whippany, NJ; Analysis Group, Inc., Boston, MA; Duke University Medical Center, Durham, NC; Carolina Urologic Research Center, Myrtle Beach, SC
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020
References:
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018;378(15):1408-1418.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018 Jun 28;378(26):2465-2474.


