Questionnaires were administered at baseline, at cross-over, at the end of treatment, and either every four weeks (Functional Assessment of Cancer Therapy-Prostate [FACT-P], Patient Health Questionnaire-9 [PHQ-9]) or every 12 weeks (Montreal Cognitive Assessment [MoCA]). A change in FACT-P score of more than 10 was considered clinically significant, with different changes assigned as significant for various subscales. TTQOLD was determined by time from baseline (start of first-line, start of second-line, or overall) until a clinically significant decline in FACT-P score. The design of the trial is shown below.
Response rates to all surveys were high (more than 80% for FACT-P even in second line, more than 70% for MoCA in second line). No difference was detected between either arm for TTQOLD with first-line therapy, second-line therapy, or throughout the entire treatment sequence of the trial. Regarding FACT-P subscales, Arm B was associated with statistically worse physical well-being (PWB) scale scores.
More patients had a PHQ-9 score ≥ 10 after therapy on enzalutamide, relative to abiraterone. No significant differences were detected in cognitive function as assessed by MoCA.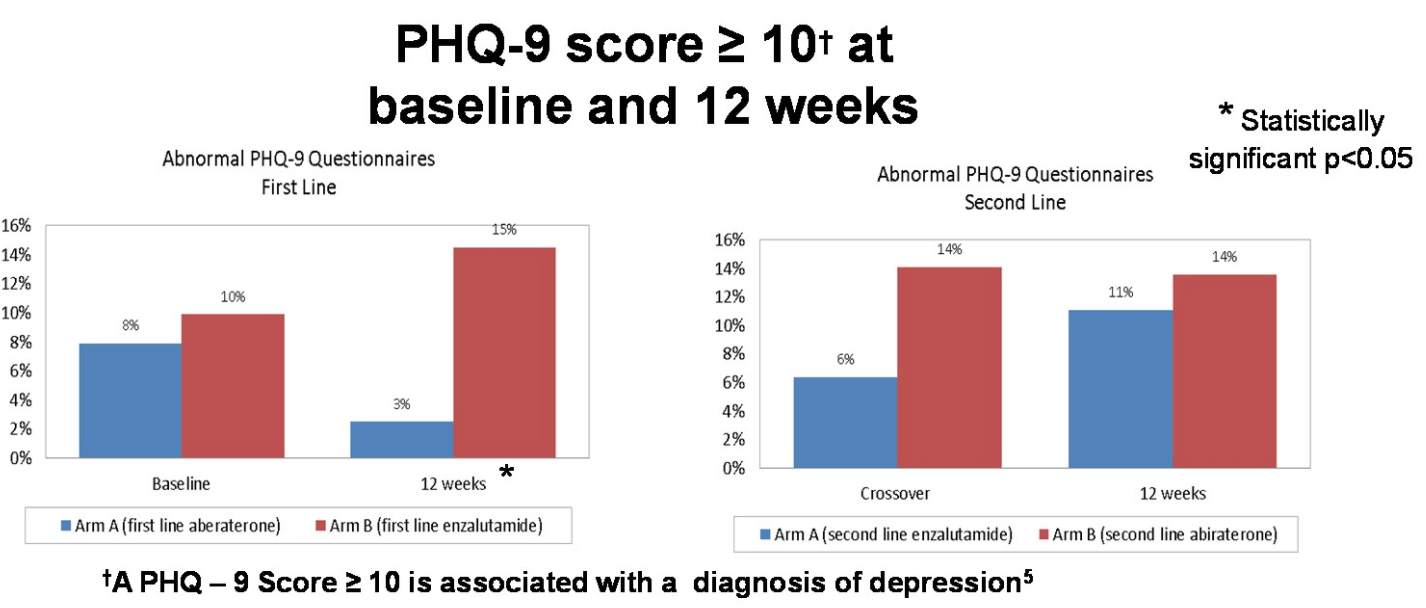
Overall, these QoL data from a randomized Phase II study suggest that treating patients with mCRPC with enzalutamide first is associated with higher PHQ-9 score and a greater decline in physical well-being, but not overall well-being or cognitive function changes.
Presented by: Daniel Joseph Khalaf, MD, Medical Oncologist, British Columbia Cancer, Vancouver, British Columbia
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, Boston, Massachusetts, at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020


