In JAVELIN Bladder 100, eligible patients had unresectable locally advanced or metastatic urothelial carcinoma without progression after 4-6 cycles of first-line gemcitabine + cisplatin or carboplatin and were randomized to receive avelumab + best supportive care (n = 350) or best supportive care alone (n = 350). The primary endpoint was overall survival (OS), in all randomized patients and patients with PD-L1+ tumors using the Ventana SP263 assay. In this exploratory analysis, Dr. Powles and colleagues analyzed OS in disease stage and site subgroups, in patients with PD-L1+ tumors who received first-line gemcitabine + carboplatin, and in genomic subtypes (RNAseq whole-transcriptome profiling of tumor tissue) defined using data from The Cancer Genome Atlas (TCGA 2017). Progression-free survival was assessed as a secondary endpoint.
Prolonged OS was observed in the avelumab + best supportive care arm versus the best supportive care alone arm in patients with upper or lower tract tumors, metastatic or locally advanced and unresectable disease (prior to chemotherapy), and lymph node-only disease post-chemotherapy. OS was also prolonged with avelumab + best supportive care in patients in PD-L1+ tumors who had received 1L gemcitabine + carboplatin, consistent with findings in the overall population:
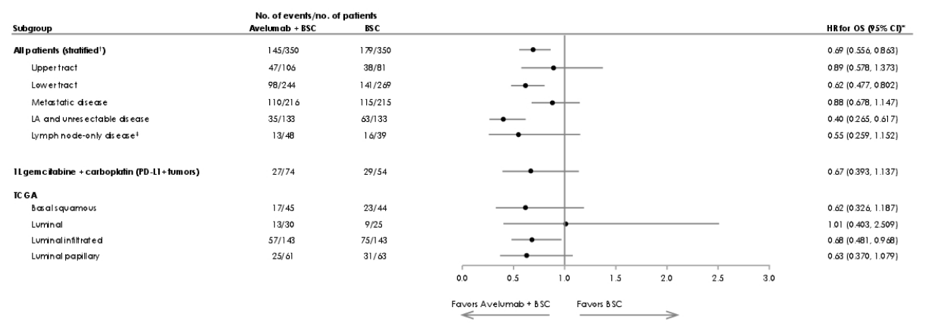
A similar trend seen in the OS analysis was reported for PFS:

In genomic subtypes, the OS benefit for avelumab + best supportive care was apparent across TCGA subtypes except luminal:
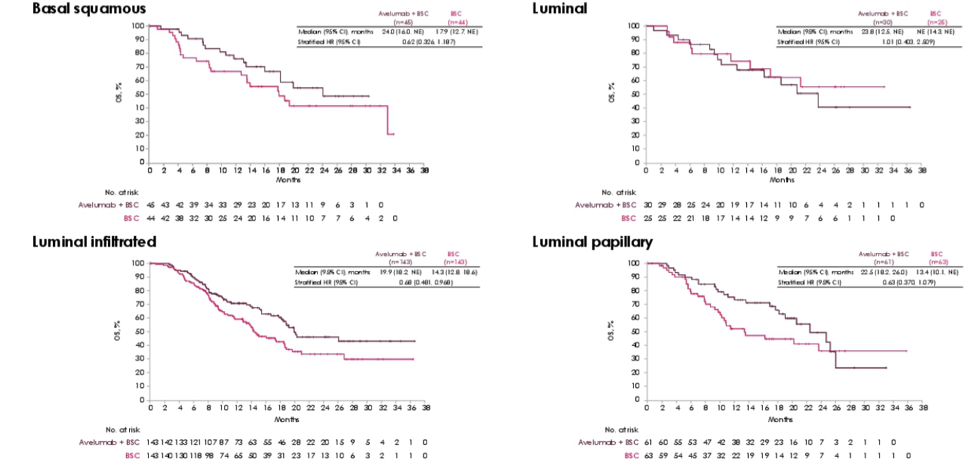
Dr. Powles concluded his presentation of posthoc analysis of the JAVELIN Bladder 100 trial with the following take-home messages:
- An OS and PFS benefit was seen for avelumab first-line maintenance + best supportive care versus best supportive care alone across subgroups of interest, including patients with upper or lower tract disease, metastatic disease, unresectable locally advance disease, or lymph node-only disease, PD-L1 positive tumors who received first-line gemcitabine + carboplatin, and tumor genomic subtypes defined by the TCGA (except luminal)
- Results are consistent with previously reported findings, further supporting avelumab first-line maintenance as a standard of care for patients with advanced urothelial carcinoma that has not progressed with first-line platinum-containing chemotherapy
Clinical trial information: NCT02603432
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:


