In JAVELIN Bladder 100, eligible patients had unresectable locally advanced or metastatic urothelial carcinoma without disease progression with 4 to 6 cycles of first-line gemcitabine + either cisplatin or carboplatin. The primary endpoint was OS from randomization, assessed in two populations: all patients and patients with PD-L1+ tumors based on the Ventana SP263 assay. In this exploratory analysis, time to the end of next-line therapy was defined in all patients as the time from randomization until discontinuation of next treatment received after first progression, as assessed by the investigator, or death from any cause, whichever occurred first. Time to next-line of therapy was analyzed as an alternative to PFS2, which could not be analyzed because progression on next-line therapy was not assessed in the trial population. The JAVELIN Bladder 100 trial schema is as follows:
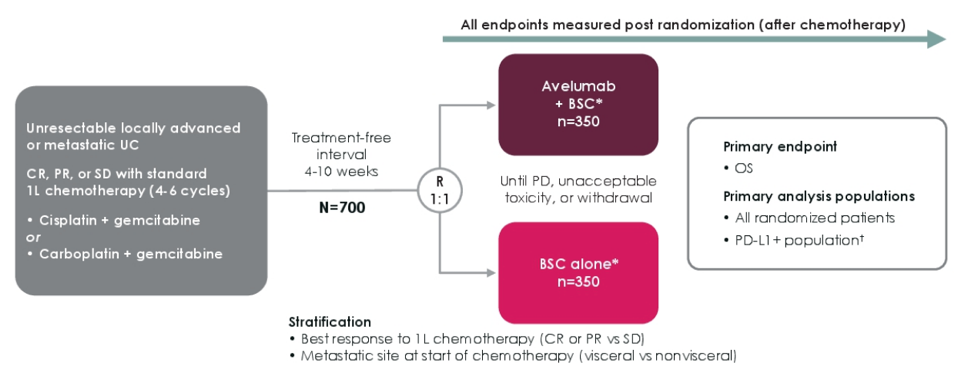
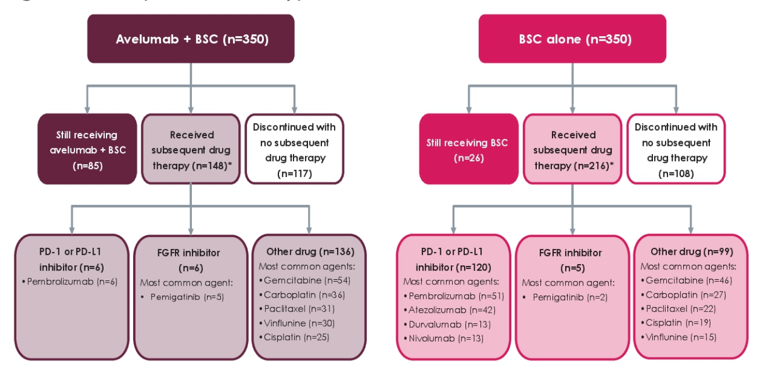
There were a total of 700 patients randomized 1:1 to avelumab first-line maintenance + best supportive care or best supportive care alone. A summary of the next line of therapy is as follows:
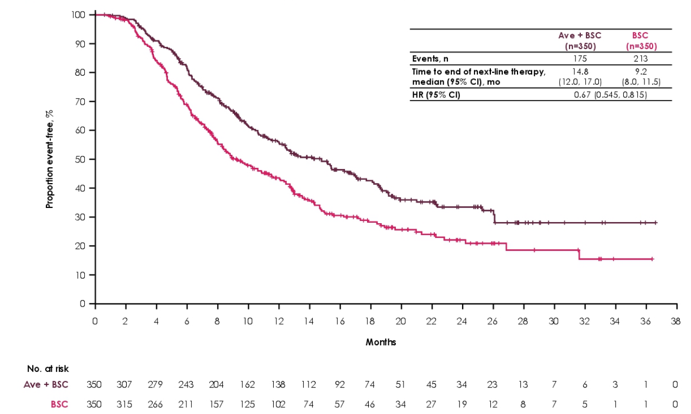
Among all randomized patients, time to end of next-line therapy was prolonged in the avelumab + best supportive care arm (median 14.8 months) versus the best supportive care alone arm (median 9.2 months): HR 0.67, 95% CI 0.545 to 0.815):
Time to end of next-line therapy was also longer in the avelumab + best supportive care arm versus the best supportive care alone arm in patients with PD-L1+ tumors (n = 358) or PD-L1− tumors (n = 270):
- PD-L1+: 18.1 versus 9.0 months, HR 0.61, 95% CI 0.451-0.818
- PD-L1-: 11.9 versus 9.3 months, HR 0.76, 95% CI 0.560-1.035
Time to end of next-line of therapy by subgroups is summarized in the following forest plot:

Dr. Grivas concluded this subgroup analysis of the JAVELIN-Bladder 100 trial with the following concluding statements:
- Patients who received avelumab first-line maintenance + best supportive care had prolonged time to end of next-line treatment compared with those who received best supportive care alone, irrespective of PD-L1 status
- These data provide further evidence of the efficacy of a maintenance approach with avelumab in patients with advanced urothelial carcinoma that has not progressed with first-line platinum-based chemotherapy
Clinical trial information: NCT02603432
Presented by: Petros Grivas, MD, Ph.D., Associate Professor, Clinical Director of Genitourinary Cancers Program, University of Washington, Associate Member, Clinical Research Division, Fred Hutchinson Cancer Research Center
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:


