This single-arm phase II registration trial testing systemic atezolizumab (1200 mg IV) every 3 weeks for one year aimed to enroll 135 (70 CIS and 65 non-CIS) eligible patients with histologically proven BCG-unresponsive high-risk NMIBC who were unfit for or declined radical cystectomy. This assessment reported the 18-month results for all eligible patients who received at least one protocol treatment. The co-primary endpoints were pathological complete response rate at 6 months in patients with CIS, and event-free survival in all patients at 18 months using Kaplan-Meier methods, conditional on a positive CIS response rate. A sample size of 135 evaluable patients provided 93% statistical power for detecting a 30% 18-month event-free survival rate versus 20% using a one-sided alpha = 0.05. Event-free survival in the subset with Ta/T1 disease and duration of response in CIS patients were secondary endpoints.
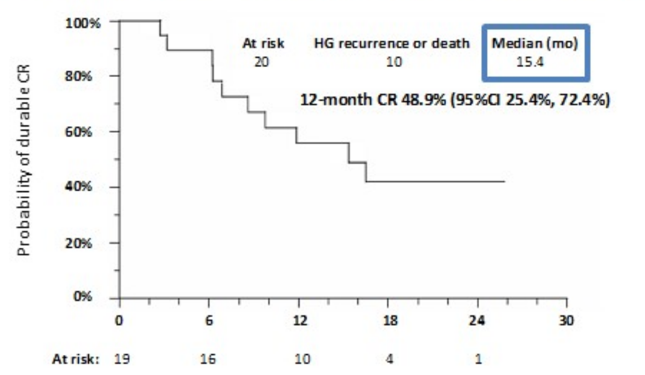
In this trial, there were 172 patients enrolled, of which 166 received at least one dose of atezolizumab and were included in the safety analysis, and, of those, 128 were eligible and included in the efficacy analysis. As previously reported, 20 (27%) out of 74 patients with CIS attained a pathologic complete response at 6 months. The Kaplan Meier estimate of 12 months (actual 11.9 months) duration of response after 6-month complete response for CIS patients was 48.9% (95% CI 25.4% to 72.4%) and the median duration of response was 15.4 months:
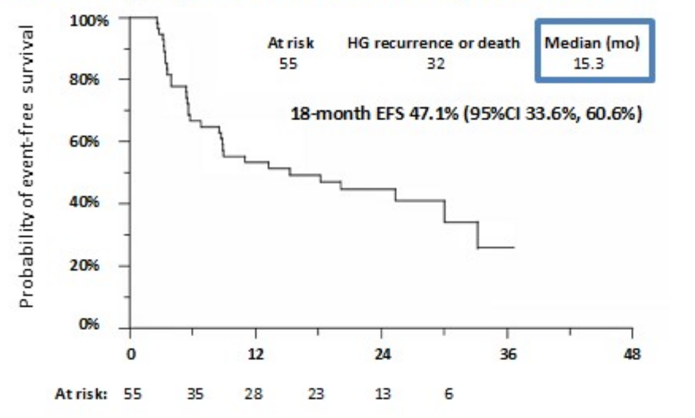
The Kaplan Meier event-free survival rate at 18 months in 74 patients with CIS was 17% (90% CI 9%, 25%), and the 18 months Kaplan Meier event-free survival rate in the overall population of 128 patients with Ta, T1, and CIS was 29% (90% CI 22%, 36%). The 18-month actuarial event-free survival rate in 55 patients with Ta/T1 disease was 47.1% (95% CI 33.6 to 60.6%):
Three patients developed muscle-invasive bladder cancer, including two at 3 months and 1 at 22 months (2 initially had CIS + high-grade T1 and one had HG Ta only). Additionally, there were two patients that developed biopsy-proven metastatic urothelial carcinoma at 17 and 31 months without recurrence in the bladder (1 HG T1 and 1 HG Ta). The number of patients proceeding to radical cystectomy and their pathologic outcomes, as well as additional therapies administered to patients after discontinuation of atezolizumab, are under review.
Any possibly or probably treatment-related adverse event was observed in 142 out of 166 (86%) patients who received any atezolizumab regardless of eligibility. The most frequent treatment-related adverse events were fatigue 72 (43%), diarrhea 34 (20%), and anemia 38 (23%). Grade 3-5 treatment-related adverse events occurred in 28 (17%) patients, including rash in 4 (2%), hyponatremia in 4 (2%), hypertension in 3 (2%) and elevated liver function tests in 3 (2%). There were two treatment-related deaths (sepsis and respiratory failure due to myasthenia gravis).
Dr. Black concluded his presentation with the following take-home messages:
- The observed complete response to atezolizumab at 6 months and the 12-month durability of complete response in patients with BCG-unresponsive CIS were similar to that of recently reported trials
- The 18-month event-free survival in patients with BCG-unresponsive Ta/T1 disease also compared favorably to recently reported results in similar patient populations
- There were no new safety concerns
- Atezolizumab demonstrates clinically meaningful activity in patients with BCG-unresponsive CIS, Ta, and T1 tumors who are ineligible for or decline cystectomy
Clinical trial information: NCT02844816
Presented by: Peter C. Black, MD, Senior Research Scientist, Vancouver Prostate Centre, Associate Director, Clinical Research, Vancouver Prostate Centre, Professor, Department of Urologic Sciences, University of British Columbia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


